Background
Pancoast tumors, first described by radiologist Henry Pancoast in the 1920s, are tumors originating in the lung apices that invade adjacent structures. They often infiltrate the eighth cervical and first and second thoracic nerve trunks and the paravertebral sympathetic chain. This results in Pancoast Syndrome, characterized by severe shoulder and arm pain, atrophy of the intrinsic hand muscles, and Horner’s Syndrome.1,2 The American College of Chest Physicians defines Pancoast tumors as primary lung cancers, whereas Pancoast Syndrome can be induced by other pathologies, including metastatic lesions, infections, and lymphoma at the lung apex.3 Pancoast tumors represent fewer than 3-5% of the estimated 45.6 cases per 100,000 people of lung cancer overall in the United States.2,4 Over 95% of Pancoast tumors are non-small cell lung cancer, with adenocarcinoma and squamous cell carcinoma being the most common.5 There are infrequent case reports of metastatic non-pulmonary malignancies mimicking Pancoast tumors and inducing Pancoast Syndrome.2 Pancoast tumors are challenging to treat due to their proximity to vital structures and are associated with significant morbidity and mortality.2,5
Primary liver malignancies are the sixth most common cancer worldwide, with hepatocellular carcinoma (HCC) comprising the majority of cases.6 Risk factors for HCC include chronic hepatitis B and C, alcohol use, non-alcoholic fatty liver diseases, and diabetes mellitus.6 Extrahepatic metastasis is estimated to occur in 14%-42% of HCC cases and portends a poor prognosis.7 While the lung is the most common extrahepatic metastatic site, there are few reports of HCC causing Pancoast Syndrome.3,6,8
Prior case reports of Pancoast Syndrome due to HCC involved older males with a significant smoking history and no history of malignancy who presented with unilateral upper extremity weakness and were found to have apical lung masses.3,8 In both cases, the patients had markedly elevated serum alfa fetoprotein (AFP) levels, as well as pathology and immunohistochemistry (IHC) results consistent with HCC. We present a different clinical scenario: a patient with a known history of unresectable HCC who developed Pancoast Syndrome due to metastatic HCC while undergoing active oncologic treatment and surveillance. We emphasize a unique aspect of the case, which was poorly differentiated pathology requiring in-situ hybridization of albumin mRNA to confirm the diagnosis of HCC. We also review the management of this rare condition.
Case Presentation
A 66-year-old male with a history of systemic lupus erythematosus on hydroxychloroquine, hepatitis C successfully treated with interferon, unresectable multifocal HCC treated with transarterial chemoembolization and radiofrequency ablation, prior tobacco use, and bilateral carpal tunnel syndrome status post left carpal tunnel release surgery presented to his rheumatologist with one year of progressive left wrist and forearm pain radiating to the shoulder. He endorsed weakness, inability to open jars, and difficulty making a fist and typing. Atrophy of the thenar compartment, decreased hand grip strength, and positive Phalen and Tinel signs were noted on exam. The initial suspected diagnosis was a recurrence of carpal tunnel syndrome (CTS). The patient was sent for a nerve conduction study, which showed evidence of proximal cervical motor axonopathy rather than median nerve abnormalities. He was subsequently referred to a neuromuscular specialist, who noted significant atrophy of the finger flexors, atrophy and fasciculations of the left arm, and no evidence of upper motor neuron disease. The patient underwent a cervical spine MRI two months after his initial presentation; this revealed a left upper lobe lung mass invading the T1 and T2 vertebral bodies and neural foramen consistent with a Pancoast tumor. The patient was then admitted to medicine for expedited oncologic evaluation.
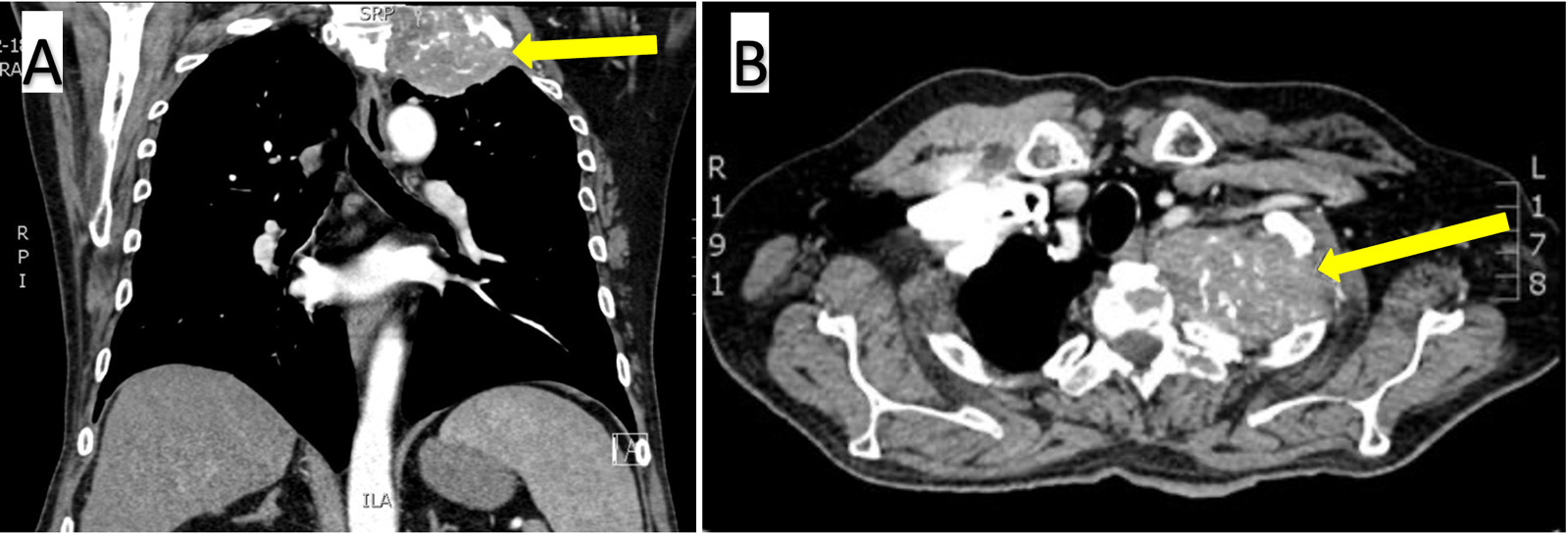
His admission exam confirmed significant atrophy of the left upper extremity and hand and the absence of Horner Syndrome. Basic labs were obtained and were unremarkable. Serum AFP was newly elevated at 16.1 (normal: <8.1). A chest CT with contrast (Figure 1), cervical spine CT, and PET scan revealed a 7.9 x 4.0 x 6.8-centimeter FDG-avid calcified mass with osseous destruction of the left first rib, invasion of the T1 and C7 vertebral bodies, and a nondisplaced pathologic fracture of the T2 transverse process. An interventional radiology-guided biopsy specimen showed poorly differentiated carcinoma containing metaplastic bone spicules (Figure 2A).
Based on the pathology report and the patient’s risk factors for lung cancer, including lupus and prior heavy tobacco use, it was unclear if the tumor was a new primary pulmonary malignancy or metastatic HCC. Immunohistochemical testing was positive for OSCAR, CK7, and CD45 and negative for TTF-1, CK20, NapsinA, PRAME, Arginase 1, CD3, CD20, synaptophysin, p63, and HepAr1. These results were not consistent with typical results for HCC or lung adenocarcinoma, and the diagnosis remained ambiguous. The tumor was then tested for albumin mRNA production and found to be positive (Figure 2B). Based on this finding and the patient’s history of unresectable HCC, the pathology team concluded that the tumor was metastatic HCC.
Oncology, thoracic surgery, and neurosurgery were consulted to determine the best treatment course. Given the extent of spinal invasion, a neurosurgical intervention was determined to be excessively risky. The patient was discharged with a plan to begin chemotherapy and radiation with palliative intent. He was treated with ten doses of radiation followed by standard chemotherapy for metastatic HCC with bevacizumab and atezolizumab. The patient received 17 cycles of chemotherapy with a plan to continue indefinitely until the development of disease progression or intolerance. At three months after treatment initiation, the patient’s AFP level had normalized, and PET-CT demonstrated partial treatment response with interval decrease in metabolic activity of the Pancoast tumor. A follow-up PET-CT performed at ten months showed stable metastatic disease without progression.
The patient suffered from significant pain related to his tumor. In consultation with palliative care, the patient was prescribed gabapentin along with methadone to achieve adequate symptom control. After a few months of chemotherapy, the patient’s pain improved, and he self-discontinued his methadone. However, his palliative care team later restarted his methadone due to recurrent pain. He remained on methadone and was living independently 18 months after the diagnosis of his lung metastasis.
Discussion
Cases of Pancoast Syndrome due to metastatic non-pulmonary malignancies are rare. A review of the literature reveals infrequent reports of isolated cases of Pancoast Syndrome secondary to metastatic malignancies of the larynx, thyroid, bladder, and cervix.2 This report is among a small number describing Pancoast Syndrome due to HCC.3,8 In reviewing this case, we discuss reasons for delays in diagnosis, the utility of albumin mRNA in the diagnosis of HCC, and management strategies for Pancoast tumors.
A critical aspect of this case is the delay in diagnosing pulmonary metastasis. The patient did not report his arm pain for over a year. This delay in seeking care was likely due to the insidious progression of symptoms, as well as deprioritization of his symptoms while addressing other urgent medical conditions. When the patient did seek care, he was sent for a nerve conduction study and to a neuromuscular specialist prior to imaging. His charted diagnosis of CTS may have led providers to assume the recurrence of a common condition, resulting in a suboptimal initial work-up. The patient was diagnosed with unresectable HCC four years prior to the identification of lung metastasis. National Comprehensive Cancer Network (NCCN) 2023 guidelines acknowledge there is limited data informing surveillance practices but recommend patients with unresectable HCC undergo cross-sectional imaging of the chest, abdomen, and pelvis every 3-6 months for two years after diagnosis followed by every six months indefinitely.9 This patient went 15 months without chest imaging despite following closely with oncology. This was likely due to recent changes in surveillance guidelines. NCCN 2021 guidelines recommended chest imaging at a more liberal interval of every 6-12 months two years after diagnosis, and prior to that, the 2019 guidelines suggested providers only “consider chest CT.”9 More aggressive surveillance imaging may have caught this patient’s pulmonary metastasis earlier. This multifactorial delay in diagnosis illustrates the importance of encouraging patients to voice their concerns, thoroughly investigating new symptoms with consideration of expedient cross-sectional imaging in patients with known malignancy and utilizing updated surveillance guidelines.
A tissue biopsy was quickly obtained after the apical lung mass was identified. However, the tumor’s poor differentiation presented challenges for the pathology team in reaching a diagnosis. While the patient had known HCC, he also had risk factors for primary lung cancer, including SLE and prior heavy tobacco use.10 The typical IHC pattern for HCC includes arginase-1 and hepatocyte paraffin antigen-1 positivity; this tumor was notably negative for both markers.8 After these equivocal IHC results, in-situ hybridization of albumin mRNA was utilized to achieve further clarity. Under normal circumstances, albumin is exclusively produced by hepatocytes, making in-situ hybridization of albumin mRNA a valuable tool in determining hepatic lineage.11 It has been increasingly used in poorly differentiated liver tumors to differentiate between primary liver neoplasms and metastases. Its use in that context has a reported sensitivity and specificity of 95% and 100%, respectively.11 However, when utilized on tumors outside the liver, there is decreased specificity. Patchy albumin mRNA positivity has been seen in gallbladder adenocarcinoma and a small percentage of other adenocarcinomas, including lung.12 Thus, while a valuable tool, it must be used in conjunction with other pathologic testing and in the context of the entire clinical picture. Ultimately, the strongly positive albumin mRNA result in the context of our patient’s known unresectable HCC helped to assure the pathology team that the diagnosis was HCC despite poor tumor differentiation and an equivocal IHC profile.
Pancoast tumors remain challenging to treat due to their anatomic location, though outcomes have improved significantly over recent decades with the refinement of surgical techniques, new oncologic drugs, and increased precision of radiation.13 Standardization of care with trimodal treatment (induction chemoradiotherapy followed by radical surgical resection) allows some patients to achieve long-term survival.13 While it is reasonable to treat metastatic apical lung lesions that mimic Pancoast tumors using a trimodal approach, there is inadequate data to prove benefit given the rarity of cases. Determining a pathologic diagnosis allows for tailored chemotherapy specific to the cancer type.
Achieving adequate pain control is essential in the comprehensive treatment of Pancoast tumors. While chemoradiation and/or surgical decompression to decrease tumor size is the mainstay of treatment, additional medications and interventions can be utilized for symptom management. Patients often suffer from severe neuropathic pain due to the compression of adjacent nerves.14 Serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and gabapentinoids are commonly employed to treat cancer-associated neuropathic pain.14 Opiates frequently are also required to achieve adequate pain control. Neuroablative procedures, localized nerve blocks, and intrathecal pump implantation should be considered for severe refractory pain.14 A combination of therapies is often necessary to achieve reasonable analgesia. Given the complexities of treating Pancoast tumors, palliative care consultation can offer valuable assistance in management. Involving palliative care early after diagnosis has been shown to improve quality of life and may even increase survival in lung cancer patients.15
In conclusion, this report describes a rare case of Pancoast Syndrome due to metastatic hepatocellular carcinoma and discusses clinical pitfalls that led to a delay in diagnosis. The case also highlights the utility of albumin mRNA in-situ hybridization in diagnosing HCC and reviews general principles in treating and managing Pancoast Syndrome.
Acknowledgements
The authors would like to acknowledge Dr. Michael Lewis (WLA VA Pathology) for his assistance in providing images of the patient’s pathology slides.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/Conflicts of Interest
The authors declare they have no conflicts of interest. The views expressed in this paper are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs or the United States government.
Corresponding author
Margaret C Slack, MD
Resident Physician
Department of Medicine, David Geffen School of Medicine at UCLA
757 Westwood Plaza, Suite 7501, Los Angeles, CA 90095
Email: MCSlack@mednet.ucla.edu
Fax: 856.212.1429

__and_positive_staining_of_albumin.png)
__and_positive_staining_of_albumin.png)