Introduction
Hospitalist care is becoming increasingly common at cancer centers where it has been shown to improve outcomes including length of stay (LOS), early discharge rate, oncologist experience,1,2 30-day readmissions2 and discharge to hospice.2,3 Hospitalists at cancer centers typically round on inpatient solid tumor services where they manage patients with acute complications resulting from cancer-directed therapy or the malignancy itself.4 Less is known about the impact of hospitalists on the care of malignant hematology patients who are electively admitted for anticancer therapy. Although cancer patients commonly receive treatment in the outpatient setting, some regimens may be administered as inpatients due to treatment complexity (e.g., etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH))5 or need for frequent monitoring for metabolite clearance (e.g., high-dose methotrexate).6 Here, we assessed the impact of hospitalist care on quality outcomes for patients with leukemia or lymphoma electively admitted for systemic anticancer therapy. We compared LOS, time from admission to anticancer therapy initiation, and discharge time on the same service during two parallel 18-month time periods prior to and following the introduction of hospitalists.
Methods
Study Design, Setting, and Participants
We performed a retrospective cohort study to compare adult patients with leukemia or lymphoma who were admitted to the Smilow Cancer Hospital for elective anticancer therapy during two separate 18-month periods. Patients who were admitted between July 1, 2018 and December 31st, 2019 were admitted to the traditional service (TS). The TS was managed by hematologists with specialization in malignant hematology. The rotating hematologists had limited inpatient responsibilities throughout the year (typically no more than 8 weeks per year), and most of their clinical time was spent in the outpatient setting. In contrast, patients who were admitted between July 26th, 2021 and December 31, 2022 were admitted to the hospitalist service (HS). The HS was managed by hospitalists with training in internal medicine who worked every other week and did not have outpatient clinics. The hospitalists were dedicated hematology-oncology hospitalists who did not attend on general internal medicine services. For both the TS and the HS, direct patient care was provided by advanced practice providers, and there was no involvement by trainees. The rounding schedule was determined by the attending physician. All queries regarding the anticancer therapy and release of orders were addressed by the patient’s primary hematologist-oncologist. The primary exposure variable for our study was the service (TS or HS).
We excluded patients who were admitted for treatment with investigational anticancer therapy regimens or hematopoietic cell transplantation, patients who received multiple anticancer therapy regimens during the included timeframe, patients treated with regimens that were exclusively administered during only one of the two time periods or in fewer than five total patients, and those with missing values for any of the covariates. The study met the Yale Institutional Review Board criteria for quality improvement; thus, no further action was required.
Covariates
Anticancer therapy regimens were dichotomized as fixed-duration and variable-duration. Fixed-duration regimens were those in which treatment was administered over a predetermined number of days and in which the inpatient stay duration was determined by this schedule. These regimens included high-dose cytarabine and dexamethasone with either cisplatin or oxaliplatin (DHAP/DHAX; 2 days); ifosfamide, carboplatin, and etoposide (ICE; 3 days); etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH; 5 days); and high-dose cytarabine (HIDAC; 5 days). Variable-duration regimens were those for which inpatient stay duration varied according to metabolite clearance, tumor lysis syndrome monitoring, or white blood cell count recovery. These included high-dose methotrexate (HD-MTX), HyperCVAD7 (due to incorporation of high-dose methotrexate in alternating cycles), liposomal daunorubicin and cytarabine (CPX-351), and venetoclax-based regimens.
Age was categorized as < 55 years, 55-69 years, and ≥70 years. Sex was binarized as male and female. The variable describing race and ethnicity was categorized as non-Hispanic White, Black and Hispanic. Cancer diagnosis discriminated among patients with lymphoma or leukemia. Severity of Illness index (SOI) is a 4-level ordinal variable derived from Diagnosis Related Groups related to patient’s primary admission diagnosis and coded co-morbidities and was binarized as SOI of 1 or 2 (less severe) and SOI of 3 or 4 (more severe).
To account for patients receiving multiple cycles of the same regimen at varying intervals during the included time frame and the potential for a cumulative effect of these, a variable representing the linear trajectory of time since the admission for the first cycle, in days, was included as a covariate. This was calculated by measuring the number of days between the start of the first cycle of chemotherapy and the start of each subsequent cycle of chemotherapy.
Outcomes
The study outcomes were LOS, time from admission to anticancer therapy, and discharge time. LOS was defined as the number of hours between admission to the hospital and discharge from the hospital and reported in days. Time from admission to anticancer therapy initiation was defined as the number of hours between admission to the hospital and first administration of fluids or medications (including pre-medications). Discharge time was defined as the time stamp when the unit secretary removed the patient from the unit census and measured as the total number of minutes elapsed from midnight of the day of discharge. The study outcomes were extracted directly from the electronic medical record.
Statistical Analysis
Bivariate analyses between study covariates and treatment service were executed using either the nonparametric Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Study outcomes were modeled using mixed linear regression models with first-order random effects for both intercept (patient) and slope (time). LOS and time from admission to anticancer therapy were modeled using a natural logarithm transformation. Discharge time was not transformed due to normal distribution of residuals as verified by a Q-Q plot. Due to a significant interaction between the type of anticancer therapy and service, separate models were constructed for fixed-duration and variable-duration anticancer therapy regimens. The adjusted models included age, sex, race and ethnicity, cancer diagnosis, SOI, and time since first admission. The models for fixed duration anticancer therapy regimens also included the number of calendar days for therapy administration. P-values less than 0.05 were a priori considered significant. All analyses were performed in STATA Basic Edition version 18.0 (StataCorp, College Station, Texas).
Results
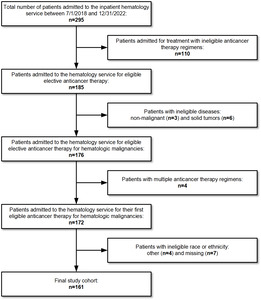
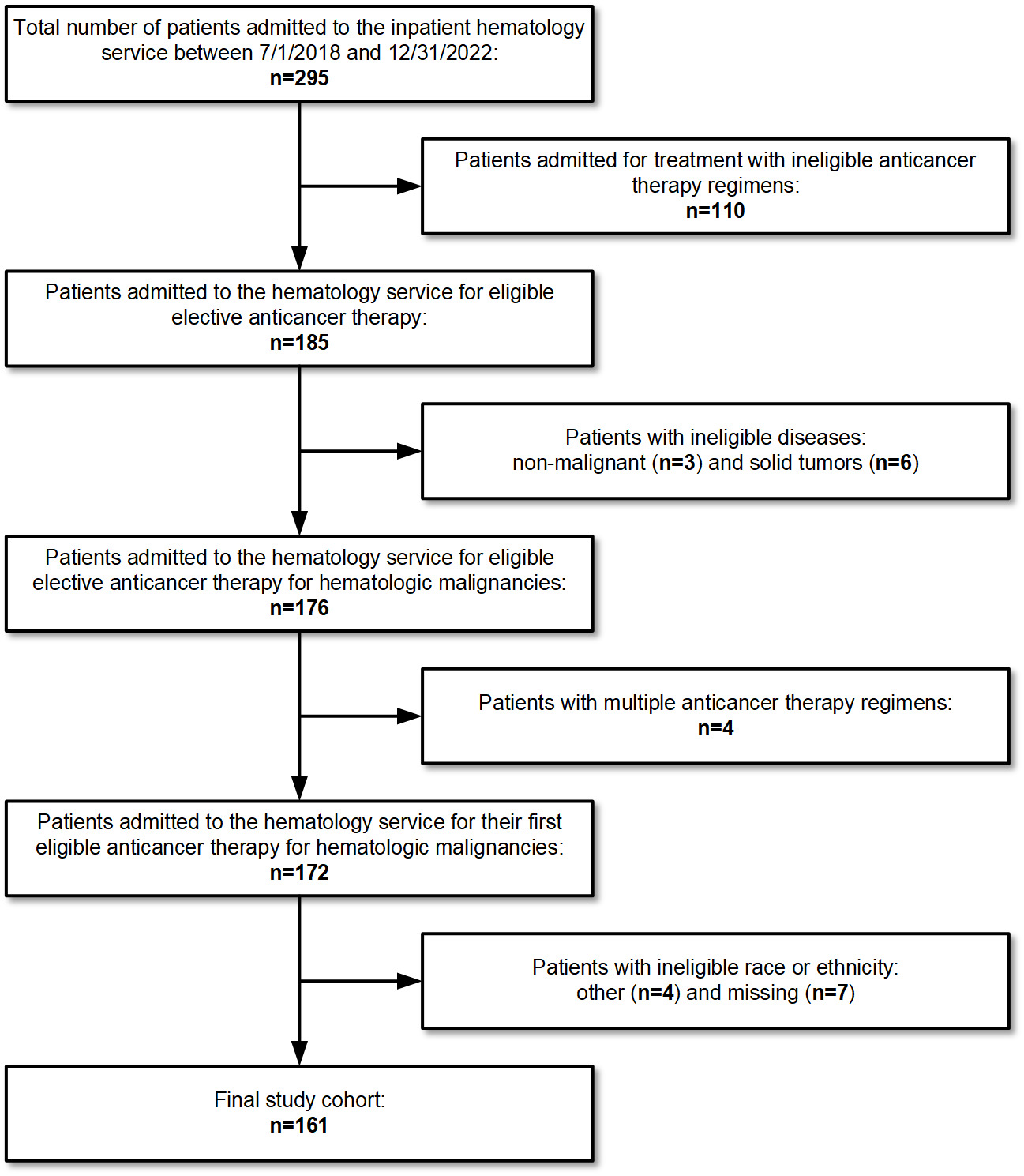
The study included 264 admissions distributed among 161 patients. There were 67 admissions among 59 patients from the TS and 197 admissions among 102 patients from the HS (Figure 1). The HS included a greater number of patients <55 years old (44% vs 28%, p=0.004). The Eastern Cooperative Oncology Group (ECOG) performance status and SOI index were time-dependent variables that changed across admissions, but there was no significant difference between services. ECOG performance status was 0-1 for 94% of admissions during the TS and 88% of admissions during the HS (p=0.38). SOI index was 1-2 for 63% of admissions during HS and 67% of admissions during the HS (p=0.55). The most common chemotherapy regimen across both services was EPOCH (27% and 30% of patients during the TS and HS, respectively). However, the overall distribution of chemotherapy regimens was significantly different between services, both in terms of the regimens themselves (p=0.03) and their expected LOS (p=0.01). Patients admitted to the TS were more likely to be admitted for their first cycle of chemotherapy (88% vs 54%, p<0.001). Admissions with an expected LOS of 5 days were more frequent for the HS (TS n=28 (42%); HS n=119 (60%); p=0.01) (Table 1).
After adjusting for age, sex, race/ethnicity, diagnosis, SOI, and time since first admission, the mean LOS for patients with fixed-duration regimens decreased from 5.97 days (95% CI: 5.13 days, 6.81 days) on the TS to 3.88 days (95% CI: 3.53 days, 4.23 days) on the HS (p < 0.001). The adjusted mean time from admission to anticancer therapy initiation among these patients decreased from 8.32 hours (95% CI: 5.72 hours, 10.93 hours) on the TS to 4.36 hours (95% CI: 3.49 hours, 5.23 hours) on the HS (p=0.001). The adjusted mean discharge time decreased by 110 minutes from 3:12 PM (95% CI: 2:06 PM, 4:19 PM) on the TS to 1:22 PM (95% CI: 12:48 PM, 1:57 PM) on the HS (p=0.01) (Table 2).
For patients who were admitted for variable-duration anticancer therapy regimens, there was no significant difference in outcomes (Table 3). For these patients, the adjusted mean LOS decreased from 6.39 days (95% CI: 4.42 days, 8.37 days) on the TS to 5.43 days (95% CI: 4.07 days, 6.78 days) on the HS (p=0.43). The adjusted mean time from admission to anticancer therapy decreased from 14.12 hours (95% CI: 7.54 hours, 20.70 hours) on the TS to 11.61 hours (95% CI: 7.93 hours, 15.29 hours) on the HS (p=0.52). The adjusted mean discharge time decreased from 3:14 PM (95% CI: 2:04 PM, 4:26 PM) on the TS to 2:41 PM (95% CI: 1:53 PM, 3:27 PM) on the HS (p=0.45).
Discussion
Reducing LOS is essential for optimizing quality of care, improving patient satisfaction, and reducing costs.8,9 This study demonstrated an adjusted mean LOS reduction among patients treated with fixed-duration anticancer therapy regimens from 5.97 days to 3.88 days after the introduction of hospitalists on an elective hematology service. This is consistent with previously published literature demonstrating the value of hospitalists to decrease LOS on inpatient general medical,10–13 surgical,14–17 and oncology services.1,2
Inpatient LOS stay is a quality indicator. Prolonged LOS is associated with increased risk of hospital acquired infections,18 thromboembolic disease,19 adverse drug reactions,20 falls,21 and mortality.22,23 Furthermore, patients who are clinically stable and have an inappropriately prolonged medical stay have an increase in both short- and long-term mortality risk.24 A meta-analysis done by the Agency for Healthcare Research and Quality (AHRQ) estimated the relative risk of mortality associated with ten common hospital acquired conditions (HACs). For the HACs reported, relative risk of mortality ranged from 1.5 (catheter associated urinary tract infection) to 3.5 (in-hospital fall).25 Any non-essential additional days spent in the hospital expose our patients to excess mortality risk. The demonstrated >2-day reduction in LOS on the HS has the potential to protect our patients from complications associated with prolonged hospital stays.
The adjusted mean time to initiation of anticancer therapy was 8.32 hours prior to hospitalist intervention and 4.36 hours after. Delay to initiation of inpatient chemotherapy has a direct impact on hospital costs and patient satisfaction, beyond that attributed to length of stay.26–29 A database study of inpatient EPOCH administration in more than 500 acute care hospitals throughout the United States found that a delay in elective chemotherapy administration of one day increased cost of care by 20%, while a delay of more than two days increased cost by 50%.8 Patients’ frustration and dissatisfaction with unnecessary delays in anticancer therapy are well-documented in the outpatient realm.30–33 Moreover, by delaying the start time of chemotherapy until the early evening, this may prompt the need for patients to stay an extra night as the last dose may finish in the late evening, making a night-time discharge less favorable to one the following morning. We found that having hospitalist availability at the time of patient arrival expedited the initial bedside evaluation. The hospitalist was able to work closely with nursing and pharmacy staff to promptly address any clinical or clerical issues prior to the start of treatment.
With emergency department (ED) overcrowding and inpatient bed shortages nationally,34–36 measures of hospital throughput, such as time of discharge, have become important patient safety metrics. Prolonged stay in the ED waiting for an inpatient bed is known to increase the risk of preventable adverse events,37 medication errors,38 and in-hospital mortality.39,40 The adjusted mean discharge time after the introduction of hospitalists to our program improved from 3:12 pm to 1:22 pm. This earlier discharge from the inpatient units is critical to ensure safe patient flow within the hospital.41,42
We report greater improvements in care quality and efficiency for patients who were admitted for regimens with defined treatment durations such as EPOCH. For patients who were admitted for regimens that required variable amounts of monitoring for post-treatment methotrexate clearance, tumor lysis syndrome, or white blood count recovery, the improvements were smaller and not statistically significant. This may be explained by the inability of attending physicians to alter how quickly patients’ clear methotrexate or recover from anticancer therapy. Interventions such as preadmission hydration and oral bicarbonate would be expected to have more impact on LOS than the specialty of the attending physician in these cases.43
Our study has several limitations. As this was a retrospective study, we were unable to control for concurrent quality improvement efforts. The inpatient pharmacy preadmission process continues to undergo revision and refinement to ensure that chemotherapy consents are completed, orders are signed, and lab tests are up to date. As these are some of the key reasons for chemotherapy delays,29 process improvement here may have a confounding effect on our study results. This was also a single-institutional study, and our results may not be generalizable to other institutions. Finally, we were not able to include all patients in our study, such as patients who received treatment on clinical trials.
Conclusions
Our results suggest that hospitalist care can facilitate improvements in quality and efficiency of elective inpatient anticancer treatment. The improved quality outcomes associated with hospitalist care may be explained by the greater availability of hospitalists compared to outpatient hematologists who face competing clinical demands. To further improve quality, safety, and patient experience, future work will need to address patient-centered and clinically safe ways to transition these anticancer treatment regimens out of the inpatient setting.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure/Conflicts of Interest
Jensa C. Morris has received honoraria from the American Board of Internal Medicine. Bonnie E. Gould Rothberg has received honoraria from MD Anderson, Cornell University, and Society of Hospital Medicine; she has served on an advisory council for Silver Hill Hospital; and she owns stock or stock options in Butterfly Networks, Abbvie, Quantum Si, Amgen, Hyperfine Research, Biocryst Pharmaceuticals, AI Therapeutics, Gilead Sciences, identifEYE, Regeneron Pharmaceuticals, Detect Labs, Roche Holdings, Protein Evolution, and Pacific Biosciences. The other authors declare no conflict of interest.
Funding Information
None
Corresponding author:
John Vaughn, MD, MS
Assistant Professor
NYU Grossman Long Island School of Medicine
120 Mineola Blvd Suite 500, Mineola, NY 11501, USA
Phone: 516-663-9500
Email: john.vaughn@nyulangone.org