Background
The rapidly changing field of immunosuppressive therapy has yielded medications such as belatacept, which have been proven more efficient and less hazardous with regard to adverse effects on kidney damage.1 Belatacept (NulojixR) is a fusion protein composed of CTLA4 and CD152 fused to the human IgG Fc portion. It produces a robust inhibition of allograft rejection by blocking both CD80 and CD86. In June 2011, the US Food and Drug Administration approved belatacept for use in combination with basiliximab induction, mycophenolate mofetil (MMF), and corticosteroids for prophylaxis of organ rejection in adult kidney transplant recipients.2 Results from Phase III BENEFIT (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial), a landmark trial in kidney allograft recipients, suggested that belatacept-based regimens are superior to calcineurin inhibitors owing to decreased toxicity, superior renal function, and improved cardiovascular profile.3 Some meta-analysis studies report similar or higher risks of allogeneic graft rejection and renal toxicity among the two.4 However, due to the immunosuppressive nature of the medication, moderate fungal infections have become evident but have been limited enough to be successfully treated. The incidence of invasive fungal infections following kidney transplant is 2.5%, significantly lower than previous studies—invasive aspergillosis accounts for most opportunistic fungal infections, followed by invasive candidiasis and mucormycosis.5,6 The risk factors associated with invasive fungal diseases (IFD) in solid organ transplant (SOT) patients, including kidney transplant recipients, include advanced age, leukopenia, diabetes mellitus, acute rejection, cytomegalovirus (CMV) infection, Tac-mycophenolate mofetil (MMF) regimen, and severe immunosuppression.5–7 The mortality rate associated with IFD in SOT patients is around 25-80% due to immunosuppression, with main risk factors including diabetes mellitus, acute rejection, deceased donor transplantation, lymphocyte-depleting antibody usage and concurrent bacterial infections.5,7 Due to the rarity of this case, we highlight a renal transplant recipient on immunosuppressive therapy with belatacept who later developed disseminated histoplasmosis and secondary hemophagocytic lymphohistiocytosis(HLH).
Case presentation
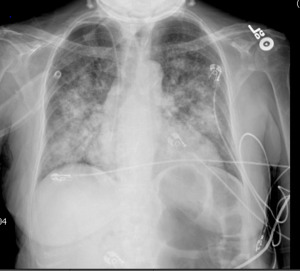
A 62-year-old female was admitted to the general medical department with fever, shortness of breath, chills, and myalgia 18 months after a deceased-donor renal transplant for end-stage renal disease from focal segmental glomerulosclerosis. The patient’s post-transplant course was complicated by gallstone pancreatitis and non-ST elevation myocardial infarction. The patient’s posttransplant immunosuppressive regimen consisted of mycophenolate 360 mg oral tablet two times daily, belatacept 250 mg IV infusion once every 30 days, and prednisone 5 mg tablet daily. The patient had received her last infusion of belatacept nine days prior to the hospital presentation. The patient’s other home medications included aspirin, atorvastatin, isosorbide mononitrate, amlodipine, sacubitril-valsartan, denosumab, pantoprazole, and minoxidil. On presentation, the patient was noted to be febrile with a temperature of 100.8 Fahrenheit, tachycardic at 102 beats per minute, and tachypneic with a respiratory rate of 32 per minute. The patient denied any history of fungal infections or recent travels and was not a resident of the histoplasmosis endemic region. Pertinent physical examination included a toxic-appearing woman in moderate distress and bilateral fine crackles in the lower lobes of the lungs. Initial laboratory workup showed an elevated procalcitonin level, lactate dehydrogenase, and transaminitis. A chest X-ray showed cardiomegaly with bilateral pulmonary infiltrates, as shown in figure 1.
The patient was admitted for acute hypoxic respiratory failure and was started empirically with vancomycin, cefepime, and doxycycline. The patient’s respiratory status continued to deteriorate despite supplemental oxygen, antibiotics, diuresis, and systemic steroids. Respiratory support was transitioned to non-invasive ventilation, and she was transferred to the intensive care unit (ICU). The patient remained critically ill in the ICU with unrelenting fevers, transfusion-dependent anemia, transaminitis, disseminated intravascular coagulation, worsening respiratory status requiring mechanical ventilation, and worsening renal function requiring dialysis. She required multiple vasopressor support to maintain the mean arterial pressure. A peripheral smear showed intracellular yeast identified by Grocott Methenamine Stain (GMS) staining in neutrophils, consistent with histoplasmosis. Additionally, degenerating fungal elements, anemia with anisopoikilocytosis, and thrombocytopenia with few giant platelets were noted as evident in Figure 2.
Bronchoscopy showed no bronchoalveolar lesions and bronchoalveolar lavage (BAL) was negative for atypical cells. In addition, BAL and urine culture were positive for Histoplasma, confirming the diagnosis of disseminated histoplasmosis. The histoplasmosis capsulatum DNA PCR in BAL was positive. Further testing was negative for COVID-19, legionella, streptococcus pneumoniae, mycoplasma pneumoniae, staphylococcus aureus, tuberculosis, pneumocystis jirovecii, and cryptococcus.
Treatment was initiated with amphotericin B, and she was transferred to a tertiary care medical center for a higher level of care. Repeat peripheral smear revealed leukocytosis with intracytoplasmic inclusions consistent with histoplasmosis. Additionally, a few degenerating fungal elements, anemia with anisopoikilocytosis, and thrombocytopenia with some giant platelets were also seen. Meropenem was initiated, and amphotericin B was continued at the tertiary center. An MRI of the brain showed focal diffusion restriction and hyperintense FLAIR signal in the posterior circulation territory and periventricular white matter of the left temporal lobe concerning for acute infarcts, small volume choroidal hemorrhage layering along the left globe. An electroencephalogram showed moderate to severe generalized nonspecific cerebral dysfunction. The patient remained on mechanical ventilation without any meaningful recovery of respiratory function. The patient’s overall clinical picture with encephalopathy, fever, and worsening pancytopenia was clinically suspicious for HLH, which was supported by hyperferritinemia (>15,000 ng/ml), hypofibrinogenemia (79 mg/dl), hypertriglyceridemia (1000 mg/dl), thrombocytopenia (88 K/Ul), anemia (6.9 g/dl), elevated IL-2 (3944.9 pg/ml), elevated LDH (>12,650) and transaminitis (AST>4070 IU/L, ALT>702 IU/L) as evident in table 1. The patient met both clinical and biological criteria for HLH and started on high-dose dexamethasone for the management of acquired HLH. Due to poor prognosis, the family opted for comfort measures, and she eventually succumbed to the disease.
Discussion
Several immunosuppressive medications have been proven to induce tolerance to allogeneic tissues by blocking T-cell activation, a significant barrier to successful transplantation. T-cell receptor (TCR) engagement and co-stimulation are essential for activating naive T cells. Blockade of costimulatory pathways has been shown to induce more robust transplant tolerance than TCR blockade alone. CD28-CD80/86 have been one of the most extensively studied costimulatory pathways in immunotherapy studies. The binding of CD28, expressed on the surface of all naïve T cells, to its ligands CD80/86 (expressed on antigen-presenting cells) leads to clonal expansion and production of cytokines. Cytotoxic T lymphocyte antigen-4 (CTLA-4, CD152), although structurally homologous to CD28, provides an inhibitory signal to T cells.8 CTLA-4 (CD 152) has a much higher affinity to CD80/CD86 than CD28. The absence of co-stimulation results in anergy and apoptosis of T-cells that underwent TCR engagement.
Although belatacept has shown long-term graft survival and an excellent safety profile regarding metabolic and cardiotoxicity, it has been associated with an increased risk of opportunistic viral infections due to its mechanism of action. In addition, belatacept has a well-known association with post-transplantation lymphoproliferative disorder in Epstein-Barr virus seronegative patients, and literature is sparse on reports of fungal opportunistic infections associated with belatacept-based therapy. Besides pneumocystis jirovecii pneumonia, belatacept has rarely been associated with severe fungal infections that necessitate treatment discontinuation.9 The previously reported case of histoplasmosis on belatacept presented mediastinal histoplasmosis that was successfully treated with amphotericin B and fluconazole without discontinuation of belatacept.1 In contrast, our patient presented with disseminated histoplasmosis and secondary HLH that resulted in the demise of the patient.
Studies show that IL 17 and other cytokines provide protective immunity against Histoplasma capsulatum.10 Mycophenolic acid and glucocorticoids have been shown to have inhibitory effects on IL 17.11 Our patient was on belatacept-based immunosuppression with mycophenolate and prednisone. Either alteration of cytokine profile by belatacept alone or increased immunosuppressive burden of belatacept in combination with mycophenolate and prednisone are favored hypotheses for fungal infection in this patient. Belatacept’s substantial suppression of T-cell activation could upset the immune system’s delicate balance, resulting in some patients’ uncontrolled activation of macrophages and other immune cells. This dysregulated immune response may contribute to the development of HLH, albeit the exact mechanism is unknown.
The patient presented in this case was diagnosed with acquired HLH towards the end of her hospital course based on characteristic clinical and laboratory findings. HLH is a life-threatening disease that is characterized by excessive immune activation. The primary form of HLH, which is commonly diagnosed in children, is caused by genetic abnormalities in the immune system. The secondary form of HLH, most commonly seen in adults, is associated with infection, malignancy, and autoimmune disorders. Although numerous organisms, including bacteria and viruses, are associated with the pathogenesis of HLH, fungi are considered more frequent causative agents for acquired HLH in immunocompromised patients.12,13 The patient presented in this case likely acquired HLH from disseminated histoplasmosis. Histoplasma-associated HLH has been associated with a high mortality rate.13–15 Therefore, early identification and prompt initiation of treatment are crucial for patient survival.
Deleterious opportunistic fungal infections like Histoplasma and the development of secondary, life-threatening diseases like HLH can outweigh the benefits of long-term graft survival by belatacept. Therefore, clinicians must maintain a high index of suspicion for fungal infections in patients who are on belatacept-based therapy.16 Further, larger-scale and post-market studies are required to assess the need for prophylactic antifungal therapy for patients on belatacept-based therapy, especially in immunologically high-risk patients with comorbidities.
Belatacept is a promising medication in the realm of immunosuppressive therapy; however, this case shows there needs to be more tailoring or augmentation of the medication with other pharmaceutical combinations to prevent disseminated fungal diseases. While this may be an isolated case with this specific patient, further research and prevention of disseminated disease is needed in future patients specifically treated with belatacept. Furthermore, clinical signs of worsening mental status, hepatosplenomegaly, pancytopenia, and elevated IL-2R in the context of disseminated fungal infections on immunosuppression or autoimmune illnesses should prompt work-up for HLH to reduce mortality.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/Conflicts of Interest
The authors declare they have no conflicts of interest
Corresponding Author
Maha Zafar MD
Department of Internal Medicine
Mercy Hospital Fort Smith
7001 Rogers ave,
Fort Smith, AR 72903
Email: maha.zafar@mercy.net
Phone: 4794619868
ORCID ID 0009-0001-6656-9262

_special_stain_that_stains_the_yeast_cell_wall_and_shows.jpeg)


_special_stain_that_stains_the_yeast_cell_wall_and_shows.jpeg)