BACKGROUND
Colchicine is a microtubule inhibitor commonly used for the treatment of gout flares. It works by binding to tubulin and inhibiting microtubule polymerization, affecting cellular processes across many organ systems.1 The most common adverse effects are gastrointestinal: diarrhea, affecting 5-10% of patients, nausea, and vomiting. In addition, colchicine has been associated with the more insidious complications of bone marrow suppression, myopathy, and neuropathy.2
Patients with renal or hepatic impairment are at increased risk of toxicity due to decreased drug elimination, leading to elevated plasma levels despite standard dosing.3 In addition, colchicine should be avoided in patients who are taking medications that are strong cytochrome P450 3A4 inhibitors or membrane P-glycoprotein drug efflux pump inhibitors, including macrolides, azoles, certain antiviral medications for human immunodeficiency virus, amiodarone, and carvedilol; these drug interactions can result in dangerously elevated colchicine levels.4 Other first-line therapies for acute gout flares include nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids. The choice of agent is often determined by patient factors that influence each drug’s relative safety profile.
CASE REPORT
A 76-year-old female presented with fatigue, generalized weakness, and diarrhea soon after starting colchicine for a gout flare. It was her first time taking colchicine, and she reported that she took two doses of 0.6 mg in the two days leading up to presentation. She developed diarrhea after the second dose, and she had 4-5 episodes of diarrhea before presenting to the hospital. At baseline she was very active, living independently and spending her free time walking or working in her garden. Since taking the colchicine, she had developed significant fatigue and generalized weakness that impaired her functioning. On review of systems, she endorsed nausea but denied any other symptoms. The patient’s past medical history was significant for stage 4 chronic kidney disease with a baseline creatinine clearance of 25, type 2 diabetes, hypertension, hyperlipidemia, gout, osteoporosis, and essential tremor. Her home medications included metformin, glipizide, empagliflozin (dose increased from 10 to 25 mg about one month prior), lisinopril, hydrochlorothiazide, amlodipine, atorvastatin, allopurinol, alendronate, vitamin D, calcium, and propranolol. Physical exam at the time of admission was significant for bradycardia and generalized weakness. Her labs were notable for elevated blood urea nitrogen and creatinine as well as hypercalcemia. Electrocardiogram (EKG) showed sinus bradycardia with a shortened QT interval.
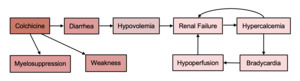
The patient’s diarrhea, likely triggered by colchicine, marked the beginning of a series of complications. The timing of onset clearly aligned with the initiation of colchicine, and the symptoms resolved within two days of discontinuing the medication. Dehydration from diarrhea, combined with the recent increase in empagliflozin dosage, resulted in acute renal failure. Records from her nephrologist showed her calcium level was 10.4 mg/dL two months prior. It was thought that the hypercalcemia to 17.5 mg/dL was the acute result of a combination of volume depletion and calcium supplementation, along with continued use of hydrochlorothiazide in the setting of renal failure. She was treated with intravenous fluids and calcitonin for her renal failure and hypercalcemia, and she received glucagon as needed for bradycardia. Her calcium level and heart rate normalized, and her renal function improved. Treatment of her gout flare was continued with prednisone with good effect.
On hospital day 3, the patient was noted to have anemia and thrombocytopenia, and this worsened, causing her to require two units of red blood cell transfusion on hospital day five and another unit on hospital day 8. Part of the acute anemia was attributed to bleeding into a superficial abdominal wall hematoma measuring approximately 5x10 cm at the site of her heparin injections for prophylaxis of deep vein thrombosis, but this alone would not be expected to account for such significant anemia or her low reticulocyte count indicating inappropriate bone marrow response to anemia. Significant gastrointestinal bleeding was ruled out as she did not have melena or hematochezia. Hemolysis and consumptive coagulopathy were ruled out based on laboratory studies. She did not have evidence of iron or vitamin B12 deficiency. Therefore, her hypoproliferative anemia and thrombocytopenia were attributed to colchicine-induced myelosuppression (Table 1). She received intravenous iron supplementation and epoetin alfa, after which her hemoglobin stabilized. Her platelet counts recovered spontaneously.
The most significant complication, which was not resolved on day of discharge, was generalized weakness most pronounced in the proximal muscles. The weakness worsened during her hospitalization, with 2/5 strength with shoulder flexion and abduction, 3/5 elbow flexion and extension, and 2/5 hip flexion and knee flexion and extension. Sensation remained intact in all extremities, and she did not complain of muscle or neuropathic pain. Her serum creatine kinase (CK) was elevated to 235 IU/L on admission. A broad differential was considered including neurologic, endocrine, and rheumatologic etiologies of weakness, and these were ruled out or considered, unlikely based on the lack of asymmetric deficits, normal thyroid stimulating hormone, absence of skin rash or other symptoms of rheumatologic disease. The weakness was attributed to colchicine-induced myopathy, and it caused significant functional impairment that persisted for the duration of her hospital stay (Figure 1). She was ultimately discharged to a skilled nursing facility with plans to engage in physical therapy with hopes of regaining her former level of functioning.
DISCUSSION
Although adverse reactions to colchicine are rare, their consequences can be substantial, especially in patients with severe CKD and other comorbidities. In this patient, diarrhea led to a dangerous cascade of renal failure, hypercalcemia, and bradycardia when combined with her CKD, recent up-titration of her diabetes medications, and use of a calcium supplement. This patient experienced the less common adverse effect of colchicine-induced myelosuppression, which is characterized by marrow aplasia beginning on day 3-5 postexposure and lasting a week or more.5 In a systematic review, the nadir hemoglobin in patients who experienced this toxicity was 9. Thrombocytopenia was not seen consistently across all studies. However, severe thrombocytopenia was seen in patients who had worsening pre-existing renal impairment or acute kidney injury, consistent with our patient’s presentation.6
While it is hard to say with certainty whether the patient’s muscle weakness was due to colchicine-induced myopathy or general deconditioning from her illness and 13-day hospitalization, the diagnosis of colchicine-induced myopathy is worth considering given the degree of weakness she developed over two days appears out of proportion to what we would expect from deconditioning in this previously very active patient. The pattern of painless proximal greater than distal muscle weakness is also consistent with colchicine-induced myopathy.7 Her concurrent use of atorvastatin may have increased her risk for this adverse effect, as there have been reports associating statins with colchicine-induced myopathy. However, in reports of colchicine-induced myopathy, CK is typically more significantly elevated to the thousands, and myopathy typically develops after prolonged use over weeks.8,9 Diagnosis is confirmed by muscle biopsy showing a vacuolar myopathy, which was not performed in this case as it would not have changed clinical management.
Considering the risk of adverse complications of colchicine in patients with renal impairment, as observed in this case, it is likely best to use glucocorticoids as a first-line agent in patients with CKD. Glucocorticoids and NSAIDs were found to be equally effective in a double-blind, randomized equivalence trial.10 However, rebound flares are common after discontinuing glucocorticoid therapy, so a gradual taper over 10-14 days is recommended if using glucocorticoids. Intraarticular glucocorticoid injection is another option that has been reported in small, open-label trials to be highly effective and would avoid the complications associated with systemic therapy. However, its safety and efficacy for acute gout have not been examined in a randomized trial, and its availability is limited by provider expertise.11
If colchicine is felt to be strongly indicated in a patient with CKD, for instance in the case of failed treatment with glucocorticoids or other contraindications to glucocorticoids, then it would be important to limit the dose and duration of treatment. Some experts recommend an initial dose of 1.2 mg followed in one hour by 0.6 mg with no repeat treatment for at least 14 days, while others recommend a more conservative initial dose of 0.3 mg with no repeat treatment for at least 3 days. Unfortunately, research into the safety of colchicine in patients with severe CKD is lacking, as most clinical trials exclude patients with CrCl < 30 mL/min.12 This patient reported taking 0.6 mg daily for two days yet still developed significant toxicity; therefore, it may be prudent to limit the dose to 0.3 mg once with no repeat treatment as per the more conservative recommendations. In addition, a cascade of complications as experienced by this patient may be prevented by counseling patients with CKD when prescribing colchicine. They should notify their provider or return to care immediately if they experience any adverse effects so that supportive care can be initiated sooner.
While it is impossible to foresee all the potential downstream complications when initiating a medication, this report highlights the importance of exercising vigilance when prescribing colchicine to patients with severe CKD.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/Conflicts of Interest
The authors declare they have no conflicts of interest
Corresponding Author
Kelly Pan, MD
Department of Medicine
Warren Alpert Medical School of Brown University
Email: kelly_pan@brown.edu