Background
Type 2 diabetes mellitus (T2DM) is a chronic condition that affects millions of patients worldwide, posing significant challenges to both patients and healthcare systems.1 T2DM remains as one of the leading causes of disability and death worldwide mainly due to its long-term vascular complications.1 The management of T2DM is becoming increasingly complex with the availability of more pharmacologic drug classes and the completion of high-quality clinical trials.2 The development of and approval of new antihyperglycemic medications has favored the use of different pharmacological families with remarkable properties for the management of additional comorbidities such as renal disease, cardiovascular disease, and non-alcoholic fatty liver disease, that are highly prevalent in this subset.2 Treatment for type 2 diabetes is initiated based on (glycated hemoglobin) HbA1C levels, treatment goals, and cardiovascular, renal, as well as hypoglycemia risk. First-line therapies, including metformin, sodium-glucose cotransporter-2 inhibitors (SGLT-2i), and Glucagon-like peptide-1 (GLP-1) receptor agonists, are selected according to patient-specific risk factors.3,4 American Association of Clinical Endocrinology guidelines recommend treatment with GLP-1RA in T2DM patients with coexisting ASCVD, high-risk for ASCVD, heart failure, or chronic kidney disease regardless of their glycemic control due to their organ protective effects.5 These guidelines align with the American Diabetes Association (ADA) standards of care which also provide strong recommendations based on robust clinical trial data supporting the cardiovascular benefits of GLP-1 RAs, particularly in patients with established cardiovascular disease and chronic kidney disease (CKD).4 For example, ADA and KDIGO joint group has recommended that for patients with T2DM and CKD who do not achieve their glycemic target with metformin and/or an SGLT2i or cannot use them, a GLP-1RAs with proven cardiovascular benefit is recommended.6 Similarly, as GLP-1RAs significantly reduced the risk of MACE, mortality from CV causes, when compared with placebo, ADA has suggested that these medications should be used in patients with underlying cardiovascular disease.4,7 In this regard, this addition ensures a more comprehensive discussion aligned with current expert consensus on risk mitigation strategies.4,6
A key issue in the physiology of type 2 diabetes (T2DM) is the reduced incretin effect, which mainly results from decreased activity of the primary incretin hormones, GLP-1 (glucagon-like peptide 1) and GIP (glucose-dependent insulinotropic peptide).8 GLP-1 is produced by L cells in the lower part of the small intestine and the colon.8 When GLP-1 receptors are activated, they promote insulin release and decrease glucagon secretion in response to glucose levels.3,8 Additional effects of GLP-1 include slowing gastric emptying, reducing appetite, and aiding in weight loss.3
The development of GLP-1 receptor agonists has shown promising results in improving metabolic markers.9 Notably, these agents have also led to a significant decrease in major cardiac events, regardless of their effect on blood glucose, indicating a dual benefit for both cardiac health and diabetes management.10 Recently, several clinical trials have shown the potential effects of these medications in not only in glycemic control but also in the improvement of cardiovascular outcomes, weight loss, osteoarthritis,11 metabolic dysfunction–associated steatohepatitis (MASH),12 Parkinson’s disease,13 chronic kidney disease (CKD)14 and obstructive sleep apnea.15 Of important interest was their effects in weight loss even in non-diabetic patients.16
Particularly the effects of these medications have covered importance in diseases closely related to obesity and DM such as CKD, cardiovascular disease and MASH. For example, the LEADER trial showed that Liraglutide significantly reduced the risk of major adverse cardiovascular events (MACE) by 13% and cardiovascular death by 22% in comparison to placebo.17 Moreover, other trials as the SUSTAIN-6 trial showed that semaglutide, another GLP-1RA also reduced the risk of MACE by 26% and non-fatal stroke by 39% in patients with T2DM.18 In the same line, the SELECT trial showed that semaglutide administered once weekly reduced the risk of MACE by 20% compared to placebo in individuals with established cardiovascular disease but no DM.16Nonetheless, other GLP-1 RAs such as albiglutide has also shown reductions on MACE risk up to 22% as demonstrated by the HARMONY trial.19 In regard to CKD, these clinical trials have also shown positive effects in the composite renal outcomes. For example, the LEADER trial showed positive renal effects with the use of liraglutide primarily by the reduction of macroalbuminuria.17 In addition to these findings, the SUSTAIN-6 trial also showed that semaglutide can offer a significant reduction in the risk of new or worsening nephropathy including persistent macroalbuminuria, increase in serum creatinine or the need of renal replacement therapy.18 Similarly, the FLOW trial proven that semaglutide showed a 24% reduction in the risk of major kidney disease events in patient with underlying T2DM and CKD.14 Finally, multiple clinical trials have demonstrated the benefits of using GLP-1RAs in MASH. For example, a phase 2 trial showed that tirzepatide, a dual Gastric Inhibitory Polypeptide (GIP) and GLP-1 RA, significantly improved MASH.20 After 52 weeks, steatohepatitis resolved without fibrosis progression in 44%, 56%, and 62% of participants receiving tirzepatide at doses of 5 mg, 10 mg, and 15 mg, respectively, compared to 10% in the placebo group.20 Similarly, A phase 2 trial of 320 patients with biopsy-confirmed MASH found that daily subcutaneous semaglutide (0.4 mg) achieved steatohepatitis resolution without fibrosis progression in 59% of patients vs. 17% with placebo, though fibrosis stage did not significantly improve.21Given this critical findings, The ADA advises using GLP-1 RAs to treat type 2 diabetes in adults with MASH, citing histological benefits demonstrated in the mentioned phase 2 randomized controlled trials.22
With the exception of oral semaglutide, available GLP-1 receptor agonists (RA) require subcutaneous injection due to their peptide-based structure.3 While effective, this method can be inconvenient for many patients and may result in lower adherence compared to oral medications.23 This need for injection can limit patient use and may also restrict the possibility of combining these drugs in fixed-dose treatments with other therapies for cardio-metabolic conditions.15,23 This issue has been addressed by the synthesis of small molecule GLP-1RA that have smaller molecular sizes and can be administered orally offering a more convenient and acceptable option to patients.24 Small molecules may offer advantages such as improved tissue permeability, longer half-lives, and lower production costs.24 In this review, we discuss the benefits and challenges associated with the small-molecule GLP-1 RA drugs from pre-clinical and clinical studies.
Methodology
We conducted a search for English-language original studies and meta-analyses published between 2010 and 2024 using PubMed, Medscape, and MEDLINE. The search included terms such as “small molecule GLP-1 receptor agonists,” “oral GLP-1 receptor agonists,” “Danuglipron,” “Orforglipron,” and “Aleniglipron.” Additionally, information on ongoing clinical trials involving small molecule GLP-1 receptor agonists was retrieved from the ClinicalTrials.gov database using the same keywords. This scoping review was carried out in accordance with the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews) guidelines.
GLP-1 and its mechanisms of regulation and synthesis
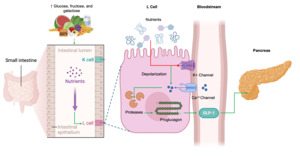
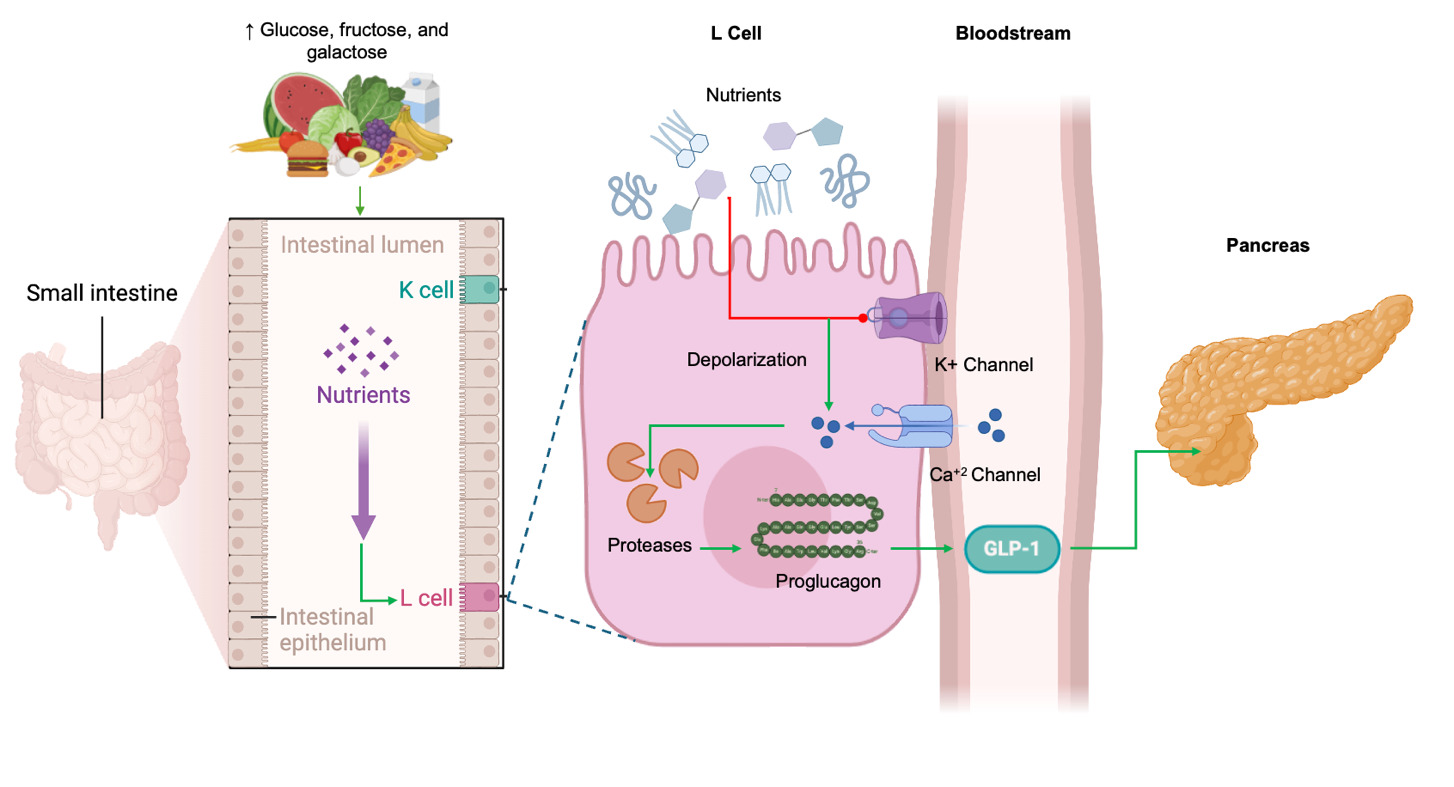
GLP-1 is a peptide hormone produced by the enzymatic breakdown of proglucagon.24 This hormone is mainly synthesized in L-cells of the intestinal lining. However, further studies have revealed that α-cells in the pancreatic islets, and neurons in the nucleus of the solitary tract are also able to produce GLP-1.25 As an endocrine hormone, GLP-1 is secreted by enteroendocrine L-cells in the distal jejunum, ileum, and colon in response to food intake and neuroendocrine signals.26 Originating from the preproglucagon precursor, it is processed in intestinal L-cells to form GLP-1.24 In this regard, prohormone convertase enzymes cleave proglucagon into several small peptides including glicentin, glicentin-related pancreatic polypeptide, glucagon, oxyntomodulin and GLP-1.27
It is well known that enteroendocrine cells produce low basal levels of GLP-1 during pre-prandial state, however after food ingestion the synthesis and secretion of GLP-1 sharply raises with a peak 30–60 min following nutrient intake.24,25,28 The L-cell’s apical surface faces the gut lumen, allowing direct contact with nutrients. Molecules like glucose, fructose, and galactose stimulate the synthesis of GLP-1 by closing ATP-sensitive K+ channels. This closure causes membrane depolarization, which opens voltage-dependent calcium channels. The influx of calcium then activates proteases that cleave proglucagon peptides, ultimately boosting GLP-1 production.27 Importantly, GLP-1 action is short-lived, with a half-life of just 1–2 minutes in the bloodstream under normal physiological conditions, after which it is broken down by the enzyme dipeptidyl peptidase IV (DPP-4), resulting in a loss of its biological activity.24,25 The mechanisms of regulation and synthesis for GLP-1secretion are summarized in Figure 1.
GLP-1R activation and multifunctional effects
GLP-1 has its main effects through the activation of GLP-1R which belongs to the family of G-protein coupled receptors.24,29 Through this receptor, GLP-1 leads to an increase of cAMP levels that thereafter activates protein kinase A (PKA).30 PKA then promotes the synthesis of insulin peptide precursor in beta-pancreatic cells and inhibits the release of glucagon by alpha pancreatic cells.31 Moreover, it has been demonstrated that GLP-1 promotes the activation of phosphoinositide 3-kinase (P13K) and protein kinase B (Akt) pathway that favor the survival and function of beta-pancreatic cells.32
GLP-1 has critical effects on alternative pathways that favor the insulin signal transduction in peripheral tissues.33 GLP-1 also reduces hepatic production of glucose by reducing the expression of key metabolic regulators of glycogenolysis. Moreover, GLP-1R cascade also has interactions to regulatory proteins involved in the insulin receptor cascades such as the insulin receptor substrates (IRS) and the PI3K involved in the positive regulation for survival, metabolism and proliferation of beta pancreatic cells as well as apical glucose transport in peripheral tissues.34 These effects are achieved by the expression and translocation of apical glucose transporter type 4 (GLUT4) thus increasing glucose uptake and by the inhibition of pro-apoptotic proteins such as Bad, Bax and FOXO family proteins, thereby improving beta cell survival.32 As previously explained, the mediator Akt is also demonstrated to activate mTOR, allowing beta cell growth through cell cycle progression and by enhancing protein synthesis.24 The main cascades involved in the signal transduction of GLP-1R are summarized in Figure 2.
GLP-1 exhibits several pleiotropic effects beyond their primary role in glycemic control. For example, studies involving GLP-1 RAs have been shown to reduce major adverse cardiovascular events in patients with T2DM.35 They have been shown to help lower blood pressure, reduce postprandial lipemia, and decrease inflammation.36 Moreover, GLP-1 RAs improve lipid profiles by decreasing serum cholesterol and triglyceride levels and increasing high-density lipoprotein (HDL) levels contributing to cardiovascular protection.37 Additionally, these agents reduce the levels of proinflammatory adipokines and the expression of inflammatory genes, which can improve overall metabolic health.24,37 GLP-1 RAs have been associated with improved renal outcomes, including enhanced natriuresis and reduced albuminuria, which are beneficial in diabetic kidney disease.38 GLP-1RAs increase microvascular blood flow in muscle tissue, enhance myocyte metabolism, inhibit muscle atrophy, and improve muscle mass and function.39 The study of these clinical properties in small molecule GLP-1RAs is an undergoing area of study.
In regard to the effects of GLP-1 in weight loss, extensive evidence demonstrates that GLP-1R agonism reduces food intake, thus promoting weight loss.40 Preclinical studies have shown that these effects are mainly mediated trough the effects of GLP-1R activation in the central nervous system (CNS) by the modulation of orexigenic and anorexigenic signaling pathways.41–44 It has been shown that GLP-1R is highly expressed in neurons of the arcuate (ARC) and paraventricular (PV) nuclei of the hypothalamus where these pathways are involved.42 The ARC contains two distinct neuron types with opposing functions. Orexigenic neurons, which stimulate appetite, produce neuropeptide Y (NPY) and agouti-related peptide (AgRP).45,46 In contrast, anorexigenic neurons suppress appetite by expressing proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) peptide.45,46 As shown in Figure 2, GLP-1RAs penetrate the blood-brain barrier and directly activate GLP-1 receptors in the hypothalamus, especially within the ARH nucleus, a key center for appetite regulation.41–44 This activation enhances the activity of POMC neurons, which induce satiety, while suppressing NPY neurons that drive hunger.41–44 Moreover, it has been shown that GLP-1RAs also promote satiety trough the activation of the nucleus accumbens by increasing glutamatergic signaling, thus suppressing feeding behavior.47
Danuglipron
Danuglipron, is a small molecule GLP-1 receptor agonist (GLP-1RA) recently discovered.3 Pre-clinical studies revealed increased insulin levels in primates.48 Initial evaluation in healthy humans revealed that oral administration of Danuglipron produced a dose-dependent increase in systemic exposure bioavailability.48 Similar results were obtained in a hGLP-1R knock-in murine model in which the administration of Danuglipron resulted in an overall improvement of glucose tolerance and food intake reduction, thus opening the possibility to use an effective oral GLP-1RA.49
Further evaluation on a double-blind, randomized phase 1 clinical trial in 98 patients with T2DM on current management with metformin revealed that Danuglipron was generally well tolerated and associated with minimal side effects such as nausea, dyspepsia, and vomiting comparable with the ones observed with the use of injectable GLP-1RA.49 In another phase 1 study conducted in 37 Japanese patients, the most common treatment-related side effects were nausea, vomiting, abdominal discomfort, diarrhea, and headache, mostly mild to moderate in severity.50 Danuglipron showed a dose-proportional increase in exposure at steady state with reductions in the mean daily glucose, fasting plasma glucose, and glycated hemoglobin (HbA1c) compared to baseline and placebo.50
Later, this medication underwent a Phase 2b clinical trial that evaluated Danuglipron in 411 adults with T2DM. The randomized, double-blind, placebo-controlled study tested various doses of Danuglipron against a placebo over 16 weeks. Participants on the highest dose (120 mg twice daily) experienced an average HbA1c reduction of 1.16 percentage points and a weight loss of 4.17 kilograms.3 All doses effectively reduced HbA1c and fasting blood glucose, with the 80 mg and 120 mg doses showing the greatest weight loss compared to placebo. The most common side effects were nausea, diarrhea, and vomiting.3 More recently, a meta-analysis integrating the available trials observed that even doses as low as 80mg twice daily were able to produce significant weight loss as well as reductions in HbA1c. However, doses of 120mg twice daily were shown to be more associated with the production of reduced appetite, dyspepsia and dizziness.51 This evidence suggest that Danuglipron demonstrates potential for glycemic control and weight reduction in T2DM with an acceptable safety profile.
Ongoing clinical trials are currently investigating a single-dose regimen of Danuglipron for managing overweight and obesity (NCT04616339). As research progresses, the initiation of a phase 3 clinical trial to further evaluate its efficacy and safety remains on the horizon, signaling the next step in its development. The most important results from the clinical studies involving danuglipron are shown in Table 1.
Orforglipron
Orforglipron, another oral GLP-1RA has being studied extensively for the management of T2DM and Obesity. In initial preclinical studies, orforglipron has been shown to stimulate insulin secretion and reduce food consumption in non-human primates, and it is fully efficacious at lowering hyperglycemia in mice expressing the human GLP-1R but inactive in GLP-1R knockout animals.52 These results, in part, supported the development of orforglipron as an orally delivered GLP-1RA in clinical testing. A Phase 1a, blinded, placebo-controlled, randomized, single- and multiple-ascending-dose study was done to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple doses of orforglipron.52 Orforglipron showed to have a pharmacodynamic and safety profile similar to that of injectable GLP-1 which supported continued clinical development.52 In a phase 1b trial done to evaluate the safety, pharmacokinetics and pharmacodynamics of orforglipron in patients with T2DM, orforglipron resulted in significant reductions in HbA1c, fasting blood glucose and body weight, with an adverse event profile consistent with that of other GLP-1RA.53 In both Phase 1a and 1b trials, it has been established that orforglipron does not have food or water administration restrictions and may provide a safe and effective oral treatment option for patients with T2D and other indications.52,53 In a phase 2, multi-center trial conducted at 45 centers, orforglipron at doses of 12 mg or greater showed significant reductions in HbA1c and bodyweight compared with placebo or dulaglutide.54 This trial emphasized that orforglipron might provide an alternative to injectable GLP-1 receptor agonists and oral semaglutide, with the prospect of less burdensome administration to achieve treatment goals in diabetics.54 In another phase 2 trial done to evaluate the efficacy of orforglipron in adults with obesity without diabetes, it was found to lead to significant weight reduction compared to placebo.55 A systematic review and meta-analysis of 7 RCT’s was done to see the efficacy and safety of orforglipron and danuglipron in the treatment of T2DM and obesity. They were found to have significant HbA1c, and weight reduction compared to placebo in patients with diabetes and obesity respectively.56 The most common adverse events were gastrointestinal in the preclinical, phase1 and 2 trials similar to trials involving injectable GLP-1 agonists.56 These trials demonstrate that although orforglipron is safe and efficacious more longitudinal research is warranted to provide deeper insights into their efficacy, safety and tolerability before their potential incorporation in the pharmacological arsenal against T2DM or obesity. The most important results of these trials are summarized in Table 2.
Aleniglipron (GSBR-1290)
Aleniglipron (GSBR-1290) is a novel oral, non-peptide GLP-1RA used in the treatment of T2DM and obesity. It has a high affinity to GLP-1R, strongly activating the GLP-1R Gαs cAMP pathway without inducing measurable β-arrestin recruitment signaling, which indicates it is a fully biased agonist. In studies involving nonhuman primates, GSBR-1290 showed a dose-dependent increase in insulin secretion, improved glucose control, and a decrease in both food intake and body weight.57 Phase 1b trial of GSBR-1290 (5–90 mg) in 24 healthy volunteers over 4 weeks demonstrated no study discontinuations due to AEs. Most AEs were mild and GI-related, consistent with GLP-1RAs.58 BW was significantly reduced (up to 4.9% placebo-adjusted, p=0.013) over 4 weeks. Phase 2a trial of GSBR-1290 (45mg and 90mg) in 54 T2DM patients over 12 weeks observed significant reduction in HbA1c (45 mg: -1.01%, p=0.008; 90 mg: -1.02%, p=0.001), BW (45 mg: -3.51%, p=0.0019; 90 mg: -3.26%, p=0.0013), and plasma glucose (45 mg: -2.70, p=0.01; 90 mg: -2.50, p=0.0008).58 Phase 2a trial of GSBR-1290 (120mg) in 40 healthy obese volunteers over 8 weeks demonstrated a significant reduction in BW (120 mg: -4.74%; p<0.0001) compared to placebo.58 For all phase 2a, AEs were mild-moderate and GI-related, with no SAEs related to GSBR-1290. The most important results from the clinical studies involving GSBR-1290 and other small molecule GLP-1RA are shown in Table 3.
Lotiglipron
The development of lotiglipron for obesity and T2DM was prematurely stopped in early-phase clinical trials due to its association with elevated transaminases and potential liver toxicity.59
Small molecule GLP-1RA in preclinical trials
MLX-7005
MLX-7005 is a highly potent, oral small-molecule GLP-1 receptor agonist (GLP-1RA) that robustly activates the GLP-1 receptor cAMP signaling pathway without measurable β-arrestin recruitment.60 In a functional human beta-cell line, it demonstrated a dose-dependent increase in insulin secretion. Preclinical studies in diet-induced obesity rodent models are ongoing to evaluate its efficacy, safety, and tolerability as a potential therapy for type 2 diabetes and obesity.60
ID110521156
ID110521156 is a small oral molecule that directly binds to the GLP-1 receptor, as demonstrated in a surface plasmon resonance assay.61 It exhibited a dose-dependent increase in intracellular cAMP levels in CHO cells expressing the human GLP-1 receptor. Once-daily oral administration of ID110521156 in diabetic monkeys resulted in reduced plasma glucose levels, increased insulin secretion, decreased food intake, and weight loss. The compound was well tolerated with no evidence of genotoxicity, indicating its potential as a therapeutic agent for type 2 diabetes and obesity in humans.61
ECC5004
The preliminary results of the investigational agent ECC5004 phase 1 trial in non-human primates revealed a reduction in HbA1c levels, accompanied by weight loss and a decreased risk of adverse cardiovascular events, achieved through the mimicry of the hormone GLP-1.62
RGT075
RGT075 is a small oral GLP-1RA identified through Computer-Accelerated Rational Design (CARD) with potent G-protein-coupled cAMP signaling.63 It displayed reduced activity in receptor-mediated β-arrestin recruitment and subsequent internalization. In food-induced glucose-intolerant and prediabetic cynomolgus monkeys, oral administration of RGT-075 improved glucose tolerance and reduced food intake and fasting glucose levels.63
Additional small molecules, including GS-4571, OWL833, and HD-7671, have demonstrated the ability to induce insulin secretion in animal models.64–66 These agents improved glucose tolerance, reduced food intake, and promoted weight loss. These preclinical findings support the further development and advancement of these small molecules as potential therapies for type 2 diabetes and obesity.
Future directions
The development of oral GLP-1 receptor agonists represents a significant advancement in the management of T2DM.9 The current advantages, research stage and areas of opportunity of small molecule GLP-1RAs is summarized in Figure 3. Overall, the newer small molecule GLP-1 RAs such as danuglipron and orforglipron have shown significant reductions in HbA1c and body weight, comparable to existing GLP-1 RAs. Injectable GLP-1 RAs like liraglutide, semaglutide, and dulaglutide have been shown to significantly lower HbA1c and body weight in patients with T2DM. Semaglutide, for example, can reduce HbA1c by up to 1.8% and weight by up to 6.5 kg.67,68 Dulaglutide and liraglutide also provide notable reductions, with HbA1c decreases of 1.0% to 1.5% and weight loss of 2–4% of total body weight.69 In comparison, newer oral small-molecule GLP-1 RAs, such as danuglipron and orforglipron, have demonstrated similar efficacy. Danuglipron reduced HbA1c by 1.04% to 1.57% and weight by 1.93 kg to 5.38 kg.49–51,56 Orforglipron achieved even greater reductions, lowering HbA1c by up to 2.1% and weight by up to 10.1 kg.55 In regard to their safety profile, small molecule GLP-1 RAs have also shown similar side effects, being the gastrointestinal symptoms such as nausea, vomiting, and diarrhea the most commonly presented. As in the case of the injectable GLP-1RAs, these side effects were generally mild to moderate and occurred during dose escalation and were generally transient and mitigated by gradual dose escalation.49,55,56,67 However, most of the clinical studies conducted up to date have focused on evaluating the effects of small molecule oral GLP-1 RAs against placebo. Further studies should now focus on non-inferiority studies comparing these new oral molecules against currently recommended injectable GLP-1 RAs to have a clear comparison between these families of medications.
However, several challenges need to be addressed to optimize their clinical utility. The future directions and challenges of new small-molecule GLP-1R agonists, such as danuglipron, are multifaceted.51 One significant challenge is the high rate of gastrointestinal adverse events associated with these compounds, which may be related to their pharmacokinetic properties and direct gastrointestinal irritation.51 Addressing these adverse effects is crucial for improving patient compliance and therapeutic outcomes.
Future directions include the design and synthesis of novel compounds that can mitigate these side effects while maintaining or enhancing efficacy. For instance, replacing problematic functional groups, such as the carboxylic acid group in danuglipron, with alternative structures has shown promise.70 For example, compound 2j, a triazole-containing derivative, has demonstrated high potency and comparable efficacy to danuglipron in preclinical studies, suggesting it could serve as a lead compound for further development.70,71 Additionally, the exploration of GLP-1R positive allosteric modulators (PAMs) offers a new regulatory approach that could enhance the therapeutic profile of small-molecule GLP-1R agonists.71 These modulators can potentially provide more selective activation of the receptor, reducing adverse effects and improving efficacy.
Finally, evaluation of small molecule GLP-1R agonists beyond their effectiveness in T2DM and obesity presents a significant area of opportunity. Other GLP-1R agonists have demonstrated extensive pleiotropic effects, including benefits in managing comorbidities such as osteoarthritis, cardiovascular disease, and neurodegenerative disorders.24 This opens the door to exploring these novel compounds as potential therapeutic agents in a broader range of conditions. Future research should prioritize investigating their role in these areas, aiming to leverage their diverse mechanisms of action for improved patient outcomes across multiple diseases.
Conclusions
Small molecule GLP-1 receptor agonists represent an innovative advancement in the management of T2DM and obesity. These compounds offer distinct advantages over their peptide-based counterparts, including oral bioavailability, improved patient adherence, and the potential for broader therapeutic applications. Their smaller molecular structure also facilitates better tissue penetration and lower production costs, which could enhance accessibility to these novel medications.
Despite these promising attributes, significant challenges remain. Ensuring efficacy, safety, and durability comparable to injectable GLP-1 RAs while minimizing adverse effects, particularly gastrointestinal discomfort, is essential. Additionally, further research is needed to explore the broader therapeutic potential of these agents in managing comorbidities such as cardiovascular disease, PCOS, CKD, MASH, and osteoarthritis. Conducting rigorous studies like those that established the benefits of injectable GLP-1 RAs such as tirzepatide, semaglutide, dulaglutide, and liraglutide on CKD, MASH, and cardiovascular outcomes will be crucial in determining the full clinical utility of these novel agents.
Small molecule GLP-RAs hold a potential to transform the landscape of metabolic and chronic disease management. Future studies will be crucial in addressing existing challenges, optimizing dosing regimens, and expanding their applications to maximize their impact on patient care.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/Conflicts of interest
The authors whose names are listed immediately below certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest) in the subject matter or materials discussed in this article.
Corresponding Author
Eder Luna-Ceron, M.D., M.Sc
Texas Tech University Health Sciences Center,
Internal Medicine Department, El Paso, Texas 79905
Email: elunacer@ttuhsc.edu