QUESTION 1: IS THERE A ROLE FOR DUAL B-LACTAM THERAPY IN MSSA BACTEREMIA?
A 31-year-old woman with a history of bipolar disorder and active polysubstance use—including fentanyl and cocaine—was admitted with a one-week history of shortness of breath, productive cough, and fever. She denied hemoptysis, back pain, or lower extremity swelling or weakness. On presentation, she was febrile to 102°F and tachycardic with a heart rate of 106 bpm. Imaging revealed a right-sided cavitary pneumonia. Empiric antibiotic therapy with IV piperacillin-tazobactam and vancomycin was initiated. Blood cultures grew methicillin-sensitive Staphylococcus aureus (MSSA), prompting a switch to cefazolin.
A transthoracic echocardiogram did not show any valvular vegetations. However, a transesophageal echocardiogram performed on hospital day 3 revealed a 2.0 cm × 0.8 cm vegetation on the tricuspid valve. Despite five days of IV anti-staphylococcal β-lactam therapy, blood cultures remained positive, and the patient continued to have intermittent fevers, although she remained non-hypoxic. Due to her ongoing refusal to engage in discussions about treatment for her intravenous drug use, thereby maintaining a high risk for reinfection, cardiothoracic surgery deemed her an unsuitable candidate for valve replacement despite persistent bacteremia.
Given her persistent bacteremia, ertapenem was added to her antibiotic regimen on hospital day 5. Surgical vegectomy by the Interventional cardiology service was considered if bacteremia failed to clear. However, following the initiation of combination therapy with ertapenem and cefazolin, blood cultures became negative within 36 hours. She was maintained on a prolonged course of antibiotics. In the following weeks, there was no evidence of invasive complications related to the bacteremia.
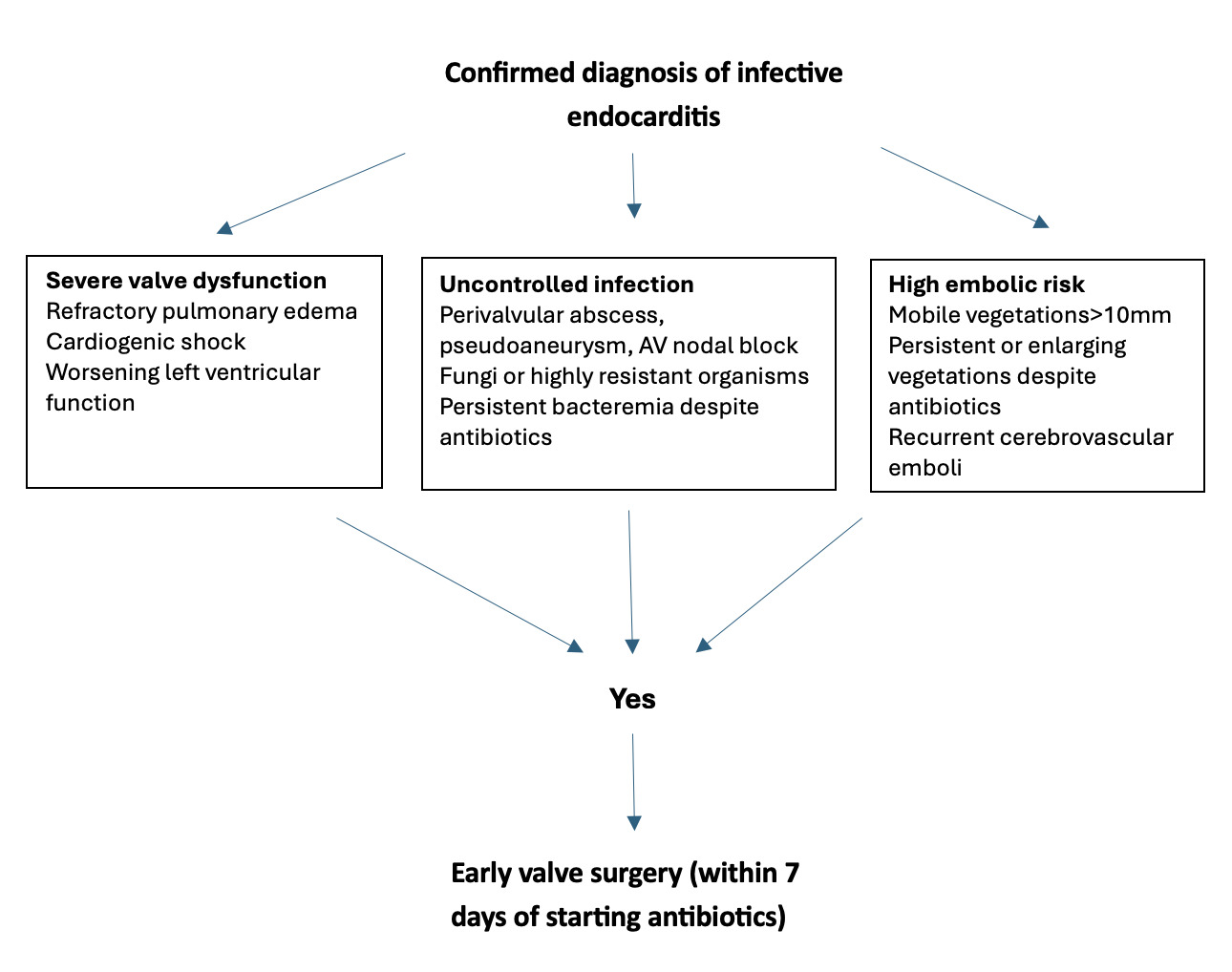
A: Methicillin-sensitive Staphylococcus aureus (MSSA) bloodstream infections are associated with substantial morbidity and mortality. Standard treatment typically involves intravenous anti-staphylococcal β-lactam antibiotics and surgical source control when appropriate. However, despite these interventions, approximately 15% of patients develop persistent bacteremia lasting more than seven days. As seen in our patient, some individuals are not suitable candidates for surgical management of infectious complications, highlighting the need for alternative therapeutic strategies to reduce adverse outcomes and mortality (Figure 1).1
The combination of ertapenem and cefazolin for the treatment of persistent MSSA bacteremia, particularly in the context of infective endocarditis, was first described in 2016.2 Since then, several case reports and meta-analyses have explored the efficacy of this dual antibiotic regimen.3 The postulated reasons for this successful management of persistent bacteremia include4:
-
Enhanced Bactericidal Activity through Complementary β-lactam Binding: The combination of two β-lactam antibiotics provides synergistic bacterial killing by targeting multiple penicillin-binding proteins (PBPs) involved in cell wall synthesis. Specifically, ertapenem and cefazolin exhibit high affinity for PBP1 and PBP2, respectively. This dual targeting may result in more complete PBP saturation, particularly at PBP1, thereby enhancing bactericidal efficacy.
-
Augmented Immune-Mediated Clearance: Exposure of MSSA to both antibiotic classes may sensitize the bacteria to the host’s innate immune responses. This synergy appears to promote increased interleukin-1β release from peripheral blood mononuclear cells, facilitating more effective immune-mediated bacterial clearance.
-
Overcoming the Eagle Effect: It has also been proposed that this dual β-lactam strategy may mitigate the “Eagle effect,” a paradoxical reduction in cefazolin efficacy at higher bacterial inocula, by enhancing the overall antibacterial activity and reducing the minimum inhibitory concentration (MIC) required for effective treatment.
In cases of persistent MSSA bacteremia beyond 72 hours, it may be reasonable to consider initiating dual β-lactam therapy. In select patients, an argument can be made for early initiation of combination therapy with escalation guided by the clinical course. While a recent meta-analysis did not demonstrate a significant difference between monotherapy and combination therapy in terms of time to blood culture clearance or 90-day mortality, the data remains limited.5 There is also a lack of robust evidence regarding the use of this dual regimen in infections involving prosthetic devices. Randomized controlled trials are needed to better assess both the clinical efficacy and potential risks of ertapenem plus cefazolin combination therapy for MSSA bacteremia.
The growing use of prosthetic devices in the management of cardiac, vascular, spinal, orthopedic, and other medical conditions has heightened concern for invasive complications in cases of persistent MSSA bacteremia, even when not primarily related to device infections. This underscores the need for a more aggressive therapeutic approach to mitigate associated morbidity and mortality. Such considerations are especially critical in patients like ours, who are not suitable candidates for surgical intervention.
Disclosures/Conflicts of Interest
The authors have no conflicts of interest to disclose.
Corresponding Author
Kwame Dapaah-Afriyie, MD
Professor of Medicine, Clinical Educator
Warren Alpert Medical School at Brown University
Division of Hospital Medicine
The Miriam Hospital, 164 Summit Avenue, Providence, RI 02906