Background
Influenza A is the seasonal cause of upper respiratory tract infection which typically presents with systemic symptoms such as fever, chills, myalgia, and malaise. The systemic symptoms of influenza are secondary to inflammatory responses and cytokine release.1 Influenza A virus attacks the upper respiratory cells and gains entry via specific hemagglutinin (HA) antigen binding to sialic acid on the cell.1 Subsequent cleavage via neuraminidase allows the spread of the virus.1 Most healthy individuals typically recover within 2 weeks.2 However, patients who have underlying lung diseases, or are immunocompromised are at the greatest risk of developing severe complications.1 Primary viral pneumonia is the most severe, least common of the pneumonic complications of influenza. It has a predilection for individuals with elevated left atrial pressures and chronic pulmonary disorders. We present a case of a middle-aged woman with no comorbidities who was infected with Influenza A and developed acute respiratory distress syndrome (ARDS).
Case Presentation
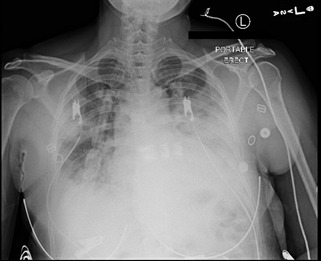
A 48-year-old woman with no past medical history presented with complaints of fever with sweats and chills, productive cough, and dyspnea with pleuritic chest pain for four days that progressively worsened to a point that she had difficulty completing sentences. She took over-the-counter nasal decongestants with no relief. On admission, vitals were significant for a temperature of 103.7 F, heart rate of 124 beats per minute, respiratory rate of 22 breaths per minute, and oxygen saturation of 92% on room air. Physical examination was significant for decreased breath sounds on the left base. A chest X-ray (Figure 1) showed left lower lobe pneumonia.
Laboratory investigations were significant for WBC of 13.5 x 103 /mm3 with 10% bands, blood and sputum cultures were drawn that remained negative, a polymerase chain reaction of nasal swab sample for methicillin-resistant staphylococcus aureus was negative. Influenza A (subtype H1) test was positive. Oseltamivir was started as well as azithromycin and ceftriaxone were started for a suspected superimposed bacterial infection. Due to persistent hypoxia, she was transferred to the intensive care unit (ICU) for oxygenation via a high-flow nasal cannula. A repeat chest X-ray (Figure 2) showed new opacities in the inferior right upper lobe and right base and small bilateral pleural effusions.
Arterial blood gas showed a pH of 7.35, pO2 of 60 mmHg, and pCO2 of 36 mmHg. During her course in the ICU, the patient’s work of breathing increased, oxygenation status continued to deteriorate, and the patient was intubated. Arterial blood gas upon intubation was consistent with severe ARDS. The patient was placed in the prone position and norepinephrine was initiated due to hemodynamic instability. Upon revaluation in the supine position, the patient was hypoxic to 88-90%, requiring positive end-expiratory pressure (PEEP) as high as 17 cm of H2O with a fraction of inspired oxygen at 100%. Due to elevated plateau pressures, the patient received paralysis but due to worsening ARDS, the patient was evaluated for venovenous extracorporeal membrane oxygenation (VV ECMO) and transferred to a tertiary care facility. The patient was cannulated and started on VV ECMO and continuous venovenous hemofiltration (CVVH) for acute kidney injury. Gradually the patient’s FiO2 requirements reduced, and she was weaned from vasopressor support, but still requiring CVVH. After 18 days of VV ECMO, a tracheostomy was performed. She tolerated daily pressure support trials and she was explanted on day 28 of ECMO. The patient remained on the ventilator for 4 more days after which she was discharged on the tracheostomy to a rehabilitation unit.
Discussion
Despite advances in critical care medicine over the past 20 years, ARDS mortality remains at 30% to 40%.1 In Australia and New Zealand, during the 2009 influenza A (H1N1) epidemic, 68 of 201 mechanically ventilated patients received ECMO across 15 ECMO centers.2 Other studies showed mortality at hospital discharge may be less when ECMO is initiated within the first seven days of mechanical ventilation.3 Due to the lack of a consistent definition of failure of conventional therapy, it is not clear what defines patient selection for ECMO and how to minimize complications on treatment. Failure of conventional respiratory support may manifest as end-organ ischemia leading to the loss of many salvageable years of the life of a previously healthy patient.
Acute respiratory distress syndrome is typically managed through supportive care. Some of the treatment goals include management of hypoxemia, reduction of oxygen consumption by controlling systemic symptoms, and fluid management.2 Extracorporeal membrane oxygenation is an effective addition for oxygen exchange in patients who are in severe acute respiratory distress syndrome.2 The premise is to oxygenate blood with a gas exchanger similar to the lungs, this allows the lungs to recover and reduces further ventilatory pressure-related lung injury.3
In the determination of when to initiate ECMO use, there is no clear consensus, however, some researchers believe that initiation of ECMO treatment typically depends on extrapulmonary system function.4 Others suggest that patients who have persistently decreased pulmonary function despite high PEEP and ventilation are good candidates for ECMO.5 Specific factors such as worsening hypoxemia; defined as PaO2/FiO2 ratio <70, hypercapnia, and acidemia are poor prognostic factors and patients may benefit from ECMO.4 The mortality rate may be unchanged in patients based on increased age, underlying lung disease, and multiple organ dysfunction.5
Veno-venous (V-V) and veno-arterial (V-A) ECMO are the two types of ECMO treatment. Veno-venous ECMO treatment is preferred because of its reduced side effects.6 These include arterial bleeding, increased risk of thrombus formation, and mixing of oxygenated and deoxygenated blood.6 Additionally, interaction with both venous and arterial blood puts patients at risk for a pro-inflammatory state.6 Various studies have outlined factors that determine the success or failure of treatment with extracorporeal membrane oxygenation (ECMO). However, the need for strict guidelines would not only help set the criteria in various institutions but may also improve mortality rates, as more patients would be quickly identified for treatment.
As per the CESAR trial, ECMO use in the setting of severe adult respiratory failure is associated with survival improvement at 6 months compared to conventional mechanical ventilation.7 It is already known that ECMO support for ARDS due to H1N1 (as other viral pneumonia) had better results than for other causes of ARDS.8 From a practical standpoint, it has been seen that good pump flow in V-V ECMO support is needed to reach satisfactory oxygenation. This allows to set up the mechanical ventilation parameters to ‘protective ventilation’ trying to limit lung damages. There can be irreversible lung damage and low possibilities of pulmonary function recovery directly due to the absence of a protective ventilation without ECMO.9 Considering observational and randomized trials in support of ECMO, this approach has been advocated for and employed in cases of complicated H1N1 infection.10 The premise for use of ECMO have been ECMO-supported inter-hospital transfer, young age, the relatively low prevalence of comorbidities, and the likelihood for reversible lung failure.11 Quoting verbatim the Extracorporeal Life Support Organization, the main indications for ECMO are acute severe heart or lung failure with high mortality risk despite optimal conventional therapy.12 ECMO therapy may be used as an adjunct or salvage therapy and no definite conclusions can be drawn yet due to the lack of randomized trials. Initiating ECMO early once the patient has been instituted on mechanical ventilation may result in improved survival.13
Corresponding author
Monarch Shah,
Department of Internal Medicine,
Saint Peter’s University Hospital.
monarch.shah08@gmail.com
1050 George Street,
Apartment 4i,
New Brunswick, New Jersey,
08901,
USA
908-392-8598
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
No funding was obtained for this manuscript.