Introduction
Pericardial tamponade is not infrequently encountered in hospitalized patients and may be related to malignancy. Providers must be vigilant in considering differential diagnoses as well as management in patients with a history of malignancy. In patients exposed to cardiotoxic medications and radiation treatments to the mediastinum, clinical entities to consider may include chemotherapy-induced cardiomyopathy and radiation-induced constrictive pericarditis. Although pericardial lymphoma and leukemia are a rare fining, there are cases of pericardial involvement leading to life-threatening tamponade.
Thus, early recognition of pericardial tamponade includes recognition of nonspecific subacute symptoms which can be mistaken for presentations of other conditions.
Case Presentation
A 52-year-old female with a history of breast cancer treated with lumpectomy and adjuvant chemoradiation therapy presented with one month of dyspnea, intermittent nonproductive cough, and thoracic back pain which progressed in the two weeks prior to admission, in addition to night sweats. She denied weight loss, chest pain, pressure, or dyspnea on exertion. One week prior to presentation a chest radiograph showed a pulmonary infiltrate, and she was treated for suspected pneumonia with levofloxacin. Her symptoms persisted, prompting an outpatient computed tomography (CT) scan of the chest which showed a left-sided pleural effusion. She then presented to hospital for further evaluation. She denied easy bruising, bleeding, hematuria, dysuria, hemoptysis, or hematemesis.
The patient had been diagnosed with estrogen receptor positive, progesterone receptor positive, HER2 negative breast cancer 12 years prior to presentation. She underwent a right lumpectomy followed by four cycles of doxorubicin and cyclophosphamide, as well as external beam radiation therapy. She was started on tamoxifen and changed to an aromatase inhibitor soon after menopause.
On physical examination, the patient was afebrile, heart rate 122 beats per minute, blood pressure 129/74 mmHg, with oxygen saturation 96% on ambient air. She appeared comfortable, without distress. Pulmonary examination revealed diminished breath sounds in left base. Cardiac sounds were diminished, and pulsus paradoxus maneuver revealed a difference of 8 mmHg. Extremity examination revealed warm, well-perfused extremities without peripheral edema. Slight jugular venous distention was noted.
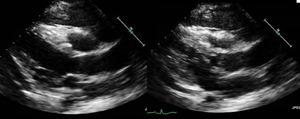
Complete blood count revealed white blood cell count 73,000 cells x 109/L with 88% blast cells, hemoglobin 10.5 g/dL, and platelets 23,000 x 109/L. Lactate dehydrogenase (LDH) 1355 IU/L and uric acid 6.2 mg/dL. ECG showed sinus tachycardia with heart rate of 109 beats per minute, poor R wave progression, and no ischemic changes. A CT scan of the chest, abdomen, and pelvis revealed an extensive anterior mediastinal mass with mediastinal lymphadenopathy, a small pericardial effusion, and large left-sided pleural effusion with pleural thickening (Figure 1). An echocardiogram showed normal left ventricular size with hyperdynamic function, mild pulmonary hypertension, elevated right-sided filling pressures, and bilateral pleural effusions. A large pericardial effusion was visualized, along with pericardial thickening. There was echocardiographic evidence of tamponade physiology with significant respirophasic variation of mitral inflow and left ventricular outflow tract outflow along with transient diastolic collapse of the RV chamber (Figure 2).
Cardiothoracic surgery performed a pericardiectomy with pericardial window placement and biopsy of an anterior mediastinal mass. 200 mL of pericardial fluid as well as 1600 mL of the left-sided pleural effusion were drained.
Pathology evaluation of the mediastinal mass demonstrated a dense, diffusely infiltrative predominantly lymphoid population. Flow cytometry revealed 84% blasts of T-cell lineage expressing CD2, CD3, CD7, and co-expression of CD4/CD8 consistent with precursor T-cell acute lymphoblastic leukemia.
The patient developed a pneumothorax requiring chest tube insertion. She was started on hydroxyurea and allopurinol, in addition to hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone) chemotherapy.
The patient completed hyper-CVAD two months after admission. She underwent salvage chemotherapy with cyclophosphamide, etoposide, and nelarabine and ultimately underwent a matched sibling donor allogeneic stem cell transplant and has been in complete remission status since.
Discussion
T-cell acute lymphoblastic leukemia is an aggressive malignancy arising from precursor T cells, characterized by ≥20% blasts in the bone marrow.1 T-ALL more commonly affects children and young adults and has a male predominance.2 In adults T-ALL represents 25% of acute lymphocytic leukemia (ALL).2 Patients usually present with lymphadenopathy or an anterior mediastinal mass that is often associated with pleural effusions. Complications of the mass may include airway obstruction, superior vena cava syndrome, and pericardial effusion. Patients can also have constitutional symptoms such as fever, sweats, weight loss and malaise. While some patients develop symptoms over weeks to months, others may present more acutely.
This case illustrates a rare complication of T-ALL with significant cardiac involvement. The likely sequence of events for this patient was the development of mediastinal T-ALL with subsequent involvement of the pericardium causing pericardial effusion with cardiac tamponade and left-sided pleural effusion. This diagnosis was initially not made due to the patient’s nonspecific symptoms of fatigue, dyspnea, and back pain, which may also be associated with subacute cardiac tamponade.
A number of imaging modalities were used to establish the diagnosis in this case. A CT scan was initially performed that showed a left-sided pleural effusion in addition to a mediastinal mass causing cardiac involvement. A transthoracic echocardiogram revealed significant respirophasic variation along with transient diastolic collapse of the right ventricle, which are signs of tamponade physiology.
Initial management of this patient was complicated by the fact that she had tumor encasing the heart. Rather than performing a pericardiocentesis to drain fluid, a pericardiectomy (pericardial “window”) was performed to facilitate biopsy of the mediastinal mass and minimize re-accumulation of pericardial fluid.
Pericardial windows are typically performed in left lateral decubitus position with single lung ventilation to increase the surface area of the pericardium. The creation of a permanent pericardial window allows for drainage into the pleural or peritoneal cavity.3 Fluid can reaccumulate in as many as 60% of cases of pericardiocentesis.4 A pericardiectomy provides almost immediate hemodynamic benefit with minimal postprocedural morbidity and mortality.5 Typically, recurrence occurs in only 5 to 10% of patients. One important consideration is performing pericardiocentesis prior to pericardial window procedure to avoid further instability or cardiovascular collapse.5–7
Prognosis in adult T-ALL varies by risk factors, patients receive chemotherapy targeted for acute lymphoblastic leukemia and may expect complete remission rates of 80-90% with 5 year disease free survival around 40-60%.8 Mediastinal tumors may recur and the line of distinction between T-ALL and an entity known as acute T-cell lymphoblastic lymphoma (T-LBL) is not sharply demarcated.9 Our patient had good response to chemotherapy followed by allogeneic bone marrow transplantation and remains disease free at the present time.
Conclusions
Patients with T-ALL commonly present with mediastinal masses and pericardial effusions. All patients with pericardial tamponade should be carefully evaluated, with consideration given to malignancy as well as other causes. Management may include surgical drainage with pericardial “window” creation and treatment of the underlying malignancy if discovered.
Corresponding Author:
Rasan Cherala, MD
Resident Physician
Department of Medicine, Warren Alpert Medical School at Brown University
The Miriam Hospital, 164 Summit Avenue, Providence, RI 02906
T: (401) 793-2104
F: (401) 793-4047
Conflicts of Interest
The authors report no conflict of interest for this work.