BACKGROUND
There have been a number of reported cases of de novo and flare-ups of glomerulonephritis after COVID-19 vaccination. Most have been associated with the mRNA vaccines such as the Pfizer-BioNTech (BNT162b2) vaccine and the Moderna (mRNA-1273) vaccine.1–3 We report a likely case of C3 Glomerulonephritis (C3GN) following vaccination with the AstraZeneca vaccine (ChAdOx1-S recombinant). We also review the literature on glomerulonephritis post COVID-19 vaccination.
CASE REPORT
A 67-year-old male presented with sudden onset of chills, leg edema that had worsened over the prior few weeks. His past medical history included psoriasis (not on oral medications), hyperlipidemia, benign prostatic hyperplasia (BPH), and hypertension (controlled on prazosin and diltiazem). He reported having mild symptoms of weakness, pitting edema, and chills that began seven months earlier. One week before presenting to the hospital, he received his first dose of the AstraZeneca COVID-19 vaccine. His vital signs at the time of admission showed blood pressure of 132/76 mmHg, with a heart rate of 88 beats/minute. His physical exam was positive for pitting edema just above the ankles. A complete blood count, thyroid stimulating hormone, and fasting glucose levels were normal. One month before his vaccination, routine blood work showed creatinine of 1.4 mg/dl (0.5-1.1 mg/dl) and trace proteinuria with no hematuria. An abdominal sonogram showed mildly increased echogenicity of both kidneys without any hydronephrosis. This was attributed to possible pre-existing hypertension.
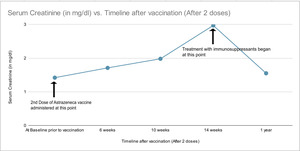
The patient’s leg edema and chills were managed with furosemide and acetaminophen by his primary care physician, with some symptomatic improvement. He received the second dose of the Astra Zeneca vaccine four months later. He continued to remain on furosemide during this period. However, after the second dose, his pre-existing symptoms worsened significantly. Six weeks after the second dose of the vaccine, his serum creatinine went up to 1.7 mg/dl, and ten weeks after the second dose, his creatinine further rose to 2.0 mg/dl. A repeat renal sonogram showed similar findings as before. A repeat urinalysis was not done at that time.
Diuretics were held at this point, and one month later, his creatinine was 3.0 mg/dl, potassium was 3.8 mmol/L (3.5-5.1), and sodium was 143 mmol/L (135-144). The creatine trend over the course of his illness has been depicted in Figure 1 . Urinalysis at this time showed increased proteinuria (3+) with trace red blood cells. Urine microscopy reported few hyaline casts and no dysmorphic RBCs. His hemoglobin dropped to 10.2 g/dl (12-16g/dl). The urine spot protein to creatinine ratio was 3.6. Serologies were remarkable for elevated ANA titers, serum C3 - 91.7 mg/dl (normal >90), and C4 - 24.7 mg/dl (10-40mg/dl).
A kidney biopsy was revealed mild background interstitial inflammation with some overall proliferation in the mesangium with some expansion under light microscopy. Three glomeruli showed mesangial expansion, and two showed glomerular basement membrane thickening. Under immunofluorescence, there was moderate C3 staining in glomerular capillary walls and granular staining in blood vessel walls consistent with C3 glomerulonephritis. Congo red stain was negative. Staining for kappa and lambda light chains was negative. Under electron microscopy, there were electron-dense deposits in the mesangium but did not reveal abnormal electron-dense deposits in the basement membrane. Mass spectrometry was not done.
Post biopsy, diuretics were restarted. Mycophenolate mofetil was started at 500 mg BID and high dose prednisone 60 mg was also started One year later, his creatinine had decreased to 1.5 mg /dl, and his urine protein to creatinine ratio was 1. He remained on mycophenolate, and prednisone.
DISCUSSION
From the time the COVID-19 vaccination rollout begun, there have been 12.03 billion doses of vaccine administered worldwide. At present there are different types of vaccines; mRNA-based, viral vector-based, and others. Overall, these vaccines have excellent efficacy and safety profiles. However, as the scale of vaccination increases, glomerular disease is reported following vaccination.4,5
Most reported cases were either minimal change disease or IgA nephropathy, and far fewer cases of C3GN were reported based on a recent analysis of individual case safety reports.6 There is one case of C3GN associated with COVID-19 vaccination.7 C3 glomerulopathies are rare with an estimated incidence of 1-3 cases per million.8 The two major types are C3GN and dense deposit disease with the former being more common in older adulthood. The broad underlying pathogenesis is excess activation of the complement pathway leading to the deposition of multiple complement components within the glomerulus. A kidney biopsy is essential for diagnosis. C3 glomerulopathy can commonly be associated with monoclonal proteins.
One of the hypotheses for our patient’s presentation is that he had quiescent/stable chronic kidney disease (CKD) from C3GN, which may have been exacerbated by vaccination. The other possibility is de novo C3GN with underlying CKD from hypertension. There have been reports of glomerulonephritis after the AstraZeneca vaccine as well, but we could not find one specifically for C3GN.9 There is still insufficient evidence to show causality or even association of glomerulonephritis and COVID-19 vaccination. COVID-19 infection has been reported in association with flare-ups of IgA nephropathy and de novo collapsing glomerulopathy.10,11 The exact immunological mechanisms that link different types of glomerulonephritis to COVID-19 vaccination are unknown. With limited data available and mass immunization, it is possible these reports may be temporally coincident associations of glomerulonephritis and the COVID-19 vaccine.
Further database research may help identify those at higher risk for flares like those with underlying GNs. Data may also help healthcare teams develop protocols for close biochemical surveillance for those who may be suspected to be at high risk for flare-ups (e.g.: underlying rheumatological disorders). Our case also had a subacute course of renal illness, unlike other cases of RPGN (rapidly proliferating glomerulonephritis).12
In conclusion, providers must be aware of the reported incidence of glomerulonephritis following COVID-19 vaccination in patients presenting with acute kidney injury. Those patients with immune-mediated renal pathology must be closely monitored in the outpatient setting.
Disclosures
The authors have no conflicts of interest to disclose.
Corresponding Author
Arjun Sekar MD
Associates in Kidney Care
Des Moines, IA, USA
Email: arjun_sekar@hotmail.com
ORCID: 0000-0002-0348-5475