Introduction
Monkeypox was first described in a 9-month-old boy in the Democratic Republic of Congo in 1970.1 It is caused by a zoonotic orthopox DNA virus closely related to variola virus, the causative agent of smallpox. Sporadic outbreaks have been previously described in endemic areas in Africa. However, since May 2022, there have been over 3000 confirmed cases in multiple non-endemic countries across the globe, prompting the World Health Organization (WHO) to declare monkeypox a global health emergency.2 We describe a male patient with fever, painful lymph nodes, and vesicular rash admitted to hospital for treatment of monkeypox. We review the known literature on the management of monkeypox infection.
Case Presentation
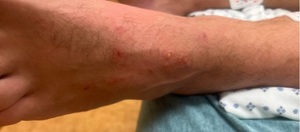
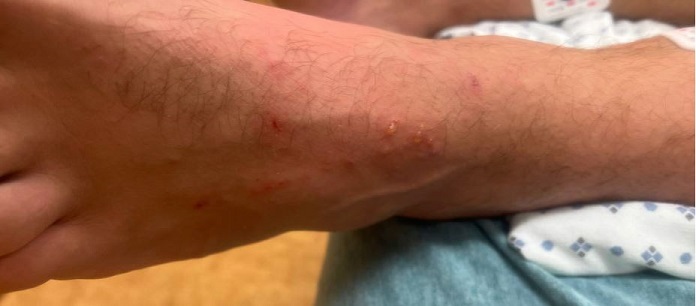
A young male in his 30s with a history of hypertension and otosyphilis presented to the hospital with a 2-day history of high fevers, chills, myalgias, fatigue, generalized weakness, and painful neck swelling. Around the same time that his symptoms developed, he noticed a new red, itchy non-painful rash on the top of his left foot and multiple raised lesions on his face and groin. The patient was diagnosed with otosyphilis and nephrotic syndrome in 2021, which resolved after treatment with intravenous penicillin. He did not take any medications at home except for pre-exposure human immunodeficiency virus (HIV) prophylaxis. He had recently traveled to a pride festival and reported having one new sexual encounter with an individual with flu-like symptoms. At the time of admission, his vital signs were blood pressure of 136/79 mmHg, temperature of 100.8° F, heart rate of 112 beats/min, and saturating well on ambient air. His skin was noted to be flushed. He had two shallow ulcers on the face that were draining purulent material (Figure 1), scattered maculopapular lesions in the back, and two pustular papules in the suprapubic region (one with umbilication), and linear punctate erythematous lesions on the left foot (Figure 2). He had enlarged bilateral anterior cervical lymph nodes and a few enlarged axillary nodes on the right side. There were no signs of photophobia, oral ulcers, conjunctival injection, or rectal pain.
His laboratory results were notable for white cell count of 11,000 (normal 4,000-11,000), C-reactive protein (CRP) 85 mg/L (0-10 mg/L), and erythrocyte sedimentation rate (ESR) 45 mm/h (0-15 mm/h). His respiratory viral pathogen panel was positive for rhinovirus/enterovirus. Pertinent negative laboratory results include negative blood parasite smear, Lyme antibodies, rickettsial antibodies, nonreactive viral hepatitis panel, and HIV. His treponemal antibody titers were >8 (0-0.8 AI). Urine analysis showed trace ketones and was otherwise normal. Swabs were obtained from his vesicular lesions and were positive for herpes simplex virus (HSV-2) by PCR testing. Swabs were also sent to the Rhode Island Department of Health laboratories for further testing and were confirmed to be positive for monkeypox virus. Given the presence of active HSV-2 lesions the patient was started on 600mg of tecovirimat (Tpoxx) twice daily for 14 days and valacyclovir 1000 mg twice daily for 10 days. Twenty-four hours later, he developed lesions on the buccal mucosa, and his pharynx was noted to be erythematous and edematous. He developed several more vesicular lesions on his back, some of which progressed to become vesiculopustular. He remained febrile for the next 48-72 hours. His symptoms were managed conservatively with antipyretic medications, intravenous fluids, and analgesics. His fever resolved 72 hours after tecovirimat initiation, and he was discharged with the plan to isolate at home until all skin lesions had crusted, sloughed and developed new skin.
Discussion
Prior to 2018, there had only been one reported monkeypox outbreak outside of the African continent.3 However, in recent months the global community has experienced a rapidly increasing incidence rate of the viral infection.2,4,5 Table 1 summarizes the clinical presentation, complications and admission criteria of monkeypox infection.
Clinical Presentation
The natural history of human monkeypox infection includes an incubation period of 10-14 days, followed by a short prodromal illness consisting of fever, malaise, and lymphadenopathy occurring in the cervical, preauricular, mandibular, inguinal, or axillary lymph nodes followed by the characteristic rash. Skin lesions are initially maculopapular and progress into papules, vesicles, pustules, and crusts, followed by desquamation and scarring over 14-21 days. Lesions are monomorphic in most patients, but a significant portion may have pleiomorphic lesions in various stages of progression. The face, trunk, limbs, hands/feet, palms/soles, and anogenital and oropharyngeal surfaces are commonly involved. Fever, lymphadenopathy, and lethargy are present in most cases, with rash/skin lesions in >90% of cases.6–8 Additional symptoms beyond rash, fever, and lymphadenopathy included headache, pharyngitis, lethargy, myalgias, low mood, and severe anorectal pain. Similar clinical findings were recorded in the 2017 outbreak in Nigeria.6
An early case series of the 2022 worldwide monkeypox outbreak revealed a predominance of cases in gay and bisexual men and men who have sex with men (GBMSM), with 41% co-infected with HIV and 29% with concomitant sexually transmitted disease.9 However the disease is not exclusive to these patients.10 Diagnosis of monkeypox virus infection may be confirmed through DNA detection in lesion scrapings, blood samples, other sites, as well as orthopoxvirus and/or monkeypox virus serologies.9 Direct viral culture of specimens may also be used though PCR testing provides the fastest results. Varicella zoster virus, HSV, Coxiella burnetii, Rickettsia, Brucella, Treponema pallidum, HIV should be considered in the differential.
Transmission
There are two major clades of the monkeypox virus, the Central African and West African clades, with the West African clade being thought responsible for the current 2022 world-wide outbreak.9 The case fatality rates vary significantly between clades: Central African clade 10.6% (95% CI: 8.4%-13.3%) vs. West African 3.6% (95% CI: 1.7%-6.8%); these numbers may be higher among immunocompromised individuals and children.11–13
While the reservoir species for monkeypox is still unidentified, there has been clear evidence historically linking wild animals (Funisciurus anerythrus, Cercocebus atys) as carriers for endemic disease.3 The current rise in cases around the globe is thought to be due to human-to-human transmission primarily via contact with skin lesions, but also possibly by large respiratory droplets, and contaminated fomites.14
Monkeypox viral DNA has been detected in seminal and vaginal fluids, but it remains unclear if the DNA found is competent for replication, and therefore it cannot be determined if the virus is transmitted sexually via semen or via the close contact of the intimate encounter.9
Admission Criteria
Patients with underlying comorbid conditions and risk factors, including immunocompromised status, are at greatest risk of severe outcomes related to monkeypox infection. Admission should be considered for patients with persistent fever, evidence of sepsis, hemodynamic instability, organ failure, skin lesions involving a large percentage of the skin surface, painful anorectal lesions, ocular involvement, and severe pharyngitis. Admission for isolation may also become a possibility, similar to the early phase of the COVID-19 pandemic, particularly for those patients living with young children, individuals who are immunosuppressed or pregnant, or have a history of atopic dermatitis placing them at higher risk.8
Inpatient Management
Infection control in health care settings is an essential component of monkeypox care. Nosocomial transmission has been reported in previous outbreaks in the Central African Republic and the United Kingdom.8 Per Centers for Disease Control (CDC) recommendations, patients should be placed in isolation rooms with dedicated bathrooms. Special air handling is not required except in case of intubation/extubation and procedures known to generate airborne particles.15 During transport through the facility, all active skin lesions should be covered with a sheet and well-fitting mask over the patient’s mouth and nose. Health care workers should don a gown, gloves, eye protection, and an N95 face mask to administer care. Isolation precautions should continue until all active pox lesions have crusted, sloughed, and formed new skin.15
The West African monkeypox clade has been widely a self-limiting disease with treatment aimed at symptomatic control. Antipyretics, intravenous fluids, and appropriate wound care for skin lesions constitute an initial approach. Disease-specific therapy should be reserved for patients with a high risk of severe outcomes. Patients with a high risk of severe outcomes include immunosuppressed individuals (including HIV/AIDS, hematological malignancy, tumor necrosis factor inhibitors, organ transplant recipients, and others), pregnant patients, history of atopic dermatitis, psoriasis, and other exfoliative dermatological diseases, pediatric populations and those with severe monkeypox disease manifestations.8 Complications of human monkeypox include secondary skin/soft-tissue infections, pneumonitis, ocular complications, and, rarely, encephalitis or death.14
After discharge, patients should isolate themselves at home and avoid close contact with others, including sexual activity. They should avoid sharing potentially contaminated items such as clothing, towels, and tableware such as plates, drinking glasses, or cutlery. Contact with pets should also be avoided as any mammal may become infected with monkeypox. In general, isolation should last until the skin lesions have lost their scabs and intact skin has formed.
Disease Specific Therapies
There are currently four disease-specific therapeutic options available for use (Table 2). Tecovirimat (TPOXX), an antiviral medication with activity against orthopox viruses, is currently recommended by the WHO and CDC for treatment, pre-exposure prophylaxis, and post-exposure prophylaxis of monkeypox infection.16 Tecovirimat inhibits the activity of orthopoxvirus VP37 protein, a viral envelope component, and prevents viral egress from the infected host cell. Tecovirimat was approved in 2018 after animal studies demonstrated efficacy against monkeypox infection.17 It is maintained in the US Strategic National Stockpile (SNS) as a safeguard against a smallpox bioterror attack. Human safety trials indicate it has few side effects and is well tolerated but no clinical studies have included children.18,19 Tecovirimat is available as an oral formulation of 600mg twice daily for 14 days upon request from the US SNS via the CDC and in an intravenous form.
Vaccinia immune globulin (VIG) is an intravenous treatment collected from pooled human plasma of individuals vaccinated against smallpox for the treatment complications of vaccinia vaccination, including eczema vaccinatum and others. Scant efficacy data are available, but this treatment may be useful for treating severely immunocompromised individuals.20
Cidofovir and brincidofovir are selective inhibitors of orthopoxvirus DNA polymerase.21 Brincidofovir was approved for the treatment of smallpox in 2021 and is an oral treatment used in several case reports of severe monkeypox disease with mixed results.16,20 Cidofovir is associated with metabolic acidosis, neutropenia, and renal failure, while brincidofovir is associated with transaminitis and hyperbilirubinemia.20
Given the paucity of human monkeypox cases, little certainty exists about outcomes. In the 2017 outbreak in Nigeria, seven deaths were reported out of 122 confirmed/probable cases (5%), including four individuals with HIV/AIDS, a post-partum woman and her newborn child.22
Special populations: Immunosuppression, Pregnancy, Pediatric
Monkeypox is suspected to produce severe outcomes in immunocompromised individuals, pregnant patients, and the pediatric population, particularly below the age of eight.23 In the 2017 outbreak in Nigeria, individuals infected with HIV-1 were more likely to have large skin lesions, genital ulcers, bacterial superinfection, and a longer duration of illness.6,22
Monkeypox was primarily a pediatric disease in the decades following its discovery, but the median age at presentation has increased over time: 4-5 years old for 1970-1989, 10 years old for 2000-2009, 21 years old for 2010-2019, and 38 years old for April-June 2022.23,24 There is very limited data in children affected by the West African clade.25
Younger children are considered to be at risk for severe outcomes from monkeypox based on early reports severe illness occurred most often in children under 10 years old.12,26,27 Furthermore, all documented deaths from monkeypox for 1970-1989 occurred in children under 10 years of age.28 In the 2003 outbreak in the USA, in which over 70% of cases occurred in adults, the only patients with serious clinical illness were children were more likely to be hospitalized in intensive care.28 As demographics have shifted over time, it is notable that 37.5% of documented deaths from monkeypox for 2000-2019 occurred in children under 10 years of age.11 Nevertheless, because data is limited overall, it is not clear whether clinical outcomes in children differ from those in adults.25
Minimal data exist on pregnancy and monkeypox, with one documented case of vertical transmission and fetal demise occurring during an outbreak in the Democratic Republic of Congo between 2007-2011.24 The safety of disease-specific therapies has not been fully evaluated in pregnancy (tecovirimat, VIG) but the U.S. Food and Drug Administration (FDA) advises seeking alternative therapies to brincidofovir. Consultation with obstetric specialists and fetal monitoring is highly recommended when caring for pregnant patients infected with monkeypox.
Role of Vaccination
The JYNNEOS vaccine (also known as Imvamune or Imvane) is one of two vaccines licensed by the FDA for the prevention of monkeypox. Past data from Africa suggests that the vaccine is at least 85% effective in preventing monkeypox. The vaccine requires two subcutaneous injections, four weeks apart.29 People are considered fully vaccinated approximately 14 days after their second shot, however, should continue to protect themselves by avoid close skin-to-skin and intimate contact with someone that has monkeypox. CDC is developing an Expanded Access Investigational New Drug protocol to allow use of JYNNEOS in children. Individuals with severe allergy to any component of the vaccine including gentamicin, ciprofloxacin and egg protein should not receive the vaccine.29 In terms of post exposure prophylaxis, the CDC recommends individuals get inoculated within four days of exposure to prevent onset of disease and no later than two weeks to reduce symptoms. The vaccine is safe for people with HIV and atopic dermatitis.
ACAM2000 is the other existing vaccine for the prevention of monkeypox.31 It is administered as a percutaneous injection via a multiple puncture technique with a bifurcated needle. Individuals are considered fully vaccinated four weeks after receiving the vaccine. Patients with cardiac disease, eye disease treated with topical steroids, congenital or acquired immune deficiency disorders, people living with HIV, atopic dermatitis/eczema, infants < 12 months of age and pregnant individuals should not get the vaccine.
Conclusions
Monkeypox infection is a disease with a wide range of symptoms and severity. Inpatients with monkeypox infection require prompt recognition, isolation and treatment strategies tailored to disease manifestations and risk factors. The knowledge base among the global community is rapidly evolving, and continued research, collaboration, and public health education will be critical in controlling the impact this virus will have on the global population.
Disclosures/Conflicts of Interest
The authors have no conflicts of interest to disclose.
Corresponding Author
Zachary Shaw
The Miriam Hospital
Division of Hospital Medicine
164 Summit Avenue
Providence, RI 02906
T: 401-793-2104
Email: zachary_shaw@brown.edu