Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to spread across the United States and globally. Similarly, the number of deaths associated with this disease continues to rise. The virus has been responsible for 6,557,284 deaths globally (https://coronavirus.jhu.edu/map.html) as of 10/09/2022. COVID-19 has a variety of clinical manifestations; from asymptomatic cases to death from acute respiratory distress syndrome and/or multiorgan failure.1,2 Convalescent plasma collected from patients who have recovered from COVID-19 was widely available in the USA as an experimental treatment for COVID-19 through an Emergency Use Authorization from the FDA.
Use of convalescent plasma therapy (CPT) stretches back over 100 years. Most recently it was used during outbreaks of similar viruses (SARS-CoV-1 and MERS-CoV) even though only a few small studies described its efficacy. One retrospective nonrandomized study with 19 patients infected with SARS-CoV-1 who received CPT demonstrated improved mortality.3 In another uncontrolled study with 80 patients infected with SARS-CoV-1 who received CPT, there was a benefit shown when the experimental drug was given early in the disease course (<14 days of illness).4
In 2020 the United States Food and Drug Administration (US FDA) issued an emergency use authorization (EUA) for the use of CPT in the treatment of COVID-19.5 At the time, early studies looking at safety showed promise but were underpowered for outcomes such as mortality, hospital duration, or ventilator free days.6–8 Additional small studies showed modest clinical benefit.9–13 The only clinical trial at the time was terminated early after only enrolling 103 patients. It did show improvement in patients considered severely ill, but for those who were considered ‘life-threatening’ (mechanically ventilated, shock, or other organ failure) it did not demonstrate any clinical improvement.14 Similar to prior experience with use of CPT in coronaviruses, early administration of CPT seemed to provide greater benefit.4,12
Since the EUA was granted, however, there have been multiple clinical trials published largely demonstrating no clinical benefit to CPT use in COVID-19.15–19 Additionally a Cochrane review published in July of 2020 including 1 RCT and multiple other studies concluded that the evidence for benefits and harms of CPT in the treatment of COVID-19 to be “uncertain.”20 A meta-analysis published in early 2021 demonstrated no mortality or clinical benefit of CPT.21
Nevertheless, there is minimal published data on the use of CPT in patients who are supported by invasive mechanical ventilation. Only two clinical trials included a significant number of intubated patients who received CPT. Neither included data regarding ventilatory parameters for subjects who receive the intervention while already intubated.15,17 In the RECOVERY trial, a subgroup analysis of patients receiving invasive mechanical ventilation favored usual care over CPT although this was not statistically significant.17 Thus far there have been only two studies that have commented on ventilatory physiologic parameters. In one study of 5 ventilated patients there was a statistically nonsignificant increase in PaO2/FiO2 ratio.6 The other was a single arm multicenter trial of CPT with 46 patients. Only 7 were intubated although all were diagnosed with moderate-severe acute respiratory distress syndrome (ARDS). This found an increase in the PaO2/FiO2 of 112 (95% CI (82,142)) for surviving patients 7 days post infusion.11 Neither study used a control arm making it difficult to determine if the intervention was responsible for these changes.
Given the rapid availability of convalescent plasma, this treatment could be deployed against future virus threats such as Ebola, SARS-CoV-1, and MERS, and it may be helpful for others to know how administration affects ventilator mechanics. Current guidelines suggest that future convalescent plasma studies be directed towards hospitalized patients with humoral immunodeficiencies, or with undetectable or low levels of anti-SARS-CoV-2 antibodies.22
Several potentially competing mechanisms for plasma-lung interaction exist. It could be speculated that the colloid volume of approximately 200 ml of plasma may, in and of itself, worsen lung compliance. Prior work has suggested an increase in lung injury score due to increased intrathoracic plasma volume after cardiac and major vascular surgery.23 Plasma therapy also carries risk of immune mediated transfusion associated lung injury (TRALI), mostly associated with donor leukocyte antibody.24
The predominant proposed protective mechanism for convalescent plasma is thought to be pathogen neutralization, although antibody-dependent cellular cytotoxicity and enhanced phagocytosis may also play a role.25 Neutralizing antibodies moderate the ongoing alveolar inflammation associated with COVID pneumonia (in fact this is how a person’s own native humoral immunity eventually clears the lung). An improvement in overall lung compliance could be reasonably proposed based on this.
Our aim is to explore changes in ventilator physiologic parameters in response to CPT transfusion using a retrospective, observational, case-control design.
Methods
Trial Design
This is a single center case control study. As this is a retrospective study, no consent was obtained or required. The research protocol including data acquisition, deidentification, storage, review, and analysis was approved by the study center institutional review board. This trial was investigator initiated with no commercial involvement.
Study Population
Patients were aged 21 years or older diagnosed with COVID-19 pneumonia confirmed by rt-PCR, intubated on mechanical ventilation and were eligible to receive CPT between March 1, 2020 and December 31, 2020. Subjects eligible to receive CPT met the following criteria: bilateral pulmonary infiltrates consistent with COVID pneumonia; respiratory failure requiring high flow supplemental oxygen, non-invasive ventilation, or mechanical ventilation; and symptom onset less than 10 days. Although the diagnosis of ARDS was not required for enrollment into the study, all participants met criteria for at least mild ARDS at the time of intubation (mean P/F ratio 122, SD 34). The decision to administer the plasma was ultimately at the discretion of the attending physician. Exclusion criteria included those patients not meeting the above inclusion criteria. Lung protective ventilator strategy with initial tidal volume (Vt) set at 6 cc/ kg ideal body weight (IBW) to Vt 8 cc/ kg IBW was the default setting used for all patients.
Trial Procedures
The study institution maintains an internal database of patients admitted to the intensive care unit. Using this database, the investigators identified the study population. Subjects given CPT were then identified as cases. Case subject characteristics were collected from the database including age, sex, and comorbidities. Additionally, a manual chart review identified the use of remdesivir and dexamethasone. Potential controls were identified via the same database. Controls were selected based on matching for age, sex, and comorbidities. A scoring system was used to match for comorbidities indicated by the Centers for Disease Control (CDC) as imparting increased risk of morbidity and mortality for COVID-19 pneumonia including reliance on hemodialysis, liver disease, chronic lung disease, diabetes mellitus (Type I and Type II), malignancy, chronic congestive heart failure, coronary artery disease, cerebral vascular disease, obesity, and history of tobacco use.26–28 One point was assigned to each comorbidity. Cases and controls were matched on this score. Additional matching was then done for the use of remdesivir and dexamethasone.
Once case subjects and potential control subjects were identified, a further manual chart review was done to collect intubation time, time of CPT administration (for cases), time of extubation (or death, whichever comes first), and in hospital mortality (yes/no). Time from intubation to CPT administration was determined for each case. Potential controls who did not remain intubated for at least the same amount of time that cases took to receive CPT (using intubation as time 0) were removed.
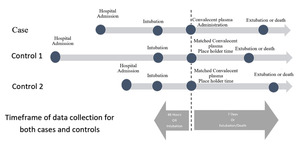
To allow concordant statistical analysis, matched controls were given a place holder time correlating to the cases actual time of CPT administration. (Figure 1). As an example, if CPT was administered 30 hours post intubation in a case, the matched controls place holder time would be intubation + 30 hours. Dependent variables (including compliance, positive end-expiratory pressure (PEEP), FiO2, tidal volume, PaO2/FiO2 ratio, and blood pressure) were extracted for all subjects. These data were collected starting 48 hours (or intubation time, if <48 hours) prior to CPT administration for cases, through 7 days post CPT administration. Similarly, data was collected starting 48 hours prior to matched control place holder time, through 7 days post place holder time. Data collection ceased if the patient was extubated or died.
Statistical Analysis
Patient characteristics were summarized as medians with interquartile ranges [IQR] for continuous data or as counts and frequencies for categorical data. We compared median differences between cases and controls with the Mann-Whitney test and differences in frequencies with the Chi-square test unless a cell count was <5, wherein the Fisher exact test was used.
To determine if the change in the physiological variables (e.g., static compliance) differed between cases and controls over time, we created panel-level random-effects linear regression models that took the repeated nature of the data over time and their sequential order within each patient (i.e., panel) into account for each physiological variable. To reduce the effect of implausible outliers, each variable had its values > 99th percentile and < 1st percentile excluded from the regression models. Predictors in each model were patient group (cases vs controls), an indicator variable for time pre vs post CPT therapy, and an interaction term for patient group and time Pre vs Post CPT. Adjusted marginal effects were then derived for the main effects and interaction term in each model along with 95% confidence intervals (CI). All p-values < 0.05 were considered statistically significant.
Results
Table 1 shows patient characteristics for the study. 12 patients received CPT while intubated (cases) and were matched 1:3 to 35 intubated control patients who did not receive CPT (1 case had only 2 matched controls). The median age of patients with and without CPT were similar: 65 vs 64 years, respectively (p=0.94). The median [IQR] APACHE IV score at the time of ICU admission was 74 [41,101] for cases and 93 [68,110] for controls (p=0.27). Median hospital day of intubation for cases was 5 days vs day 2 for controls, p= 0.22. For the 12 cases, the median [IQR] total number of days physiologic data was obtained was 8 [6, 9] days. Data collection began a median of 2 days prior to the administration of CPT. The median [IRQ] length of time from hospital admission to CPT administration was 8 [4,11] days. The controls had similar values. Table 2 shows the unadjusted median and IQR results for each physiological variable for cases and controls by Pre vs Post CPT time.
Table 3 presents the adjusted mean (marginal) effects of the physiologic variables studied, and the sample size for each variable. The variables of static compliance, plateau pressure, and pO2 required a respiratory therapist to assess and manually input ventilator data. As such, these data are obtained less frequently than data which is automatically generated and input into the hospital database such as heart rate, blood pressure, and respiratory rate. For example, 857 separate measurements of static compliance were obtained in total.
No significant difference was noted in static compliance after CPT. In cases, static compliance was 30.8 (95% CI: 23.3, 38.4) mL/cm H2O before CPT, and 28.2 (95% CI: 20.7, 35.6) mL/cm H2O afterwards. This was similar to matched controls, where static compliance was 33.9 (95% CI: 29.5, 38.4) mL/cm H2O before time-indexed ‘non-intervention’ and 32.2 (95% CI: 27.9, 36.5) mL/cm H2O afterwards. Figure 2 presents the static compliance results for cases; pre CPT static compliance is depicted as a black line and post CPT is depicted as a red line. There were no significant trends found in static compliance among cases (n.b., 3 cases [subjects #4, 7, and 8] did not survive long enough and/or have a sufficient number of static compliance measurements to report).
Table 3 does highlight in bold-face and in color the physiologic variables that had a statistically significant interaction term (i.e., the amount of change in the physiologic variable from Pre vs Post CPT time differed more than expected between cases and controls). For example, diastolic blood pressure (DBP) increased from 64.3 mmHg to 68.4 mmHg after CPT among cases while the mean DBP remained stable for controls in the pre vs post periods: 65.3 vs 65.0 mmHg (and the interaction between case vs control and pre vs post CPT was significant, with p<0.001). Similarly, systolic blood pressure (SBP) also increased from 117.3 mmHg to 125.2 mmHg after CPT for cases, while the control group remained stable: 115.3 vs 117.8 mmHg, interaction effect p = 0.001. Interestingly after CPT, tidal volume decreases from 429.5 (95% CI: 389.2, 469.8) mL to 403.0 (95% CI: 362.6, 443.3) mL, while in the control group tidal volume increased from 416.8 (95% CI: 393.1, 440.4) mL to 432.9 (95% CI: 409.3, 456.5) mL, interaction effect p<0.001. Other physiological variables that had small but statistically significant different values pre vs post CPT among cases and controls were FiO2, heart rate, applied PEEP, and respiratory rate (See Table 3).
Discussion
Our study assessed the physiologic and ventilatory variables of patients with COVID-19 pneumonia over time who received CPT compared to matched controls. We note the lack of clearly proven clinical benefit of CPT.9–13,15–19 CPT may still have some modest clinical impact as evidenced by changes in the ventilatory variables. Moreover, the use of antibody treatment, in the form of monoclonal antibodies, has seen success.
We observed small but statistically significant changes in some physiologic variables in patients who received CPT for COVID-19 pneumonia while intubated (Table 3 highlighted). Ultimately, we feel these changes to not be clinically significant or have no more than modest clinical significance. Likewise, there was no difference in secondary outcomes such as in hospital mortality, although this study was not powered for this outcome (Table 1).
We assessed multiple variables including compliance, FiO2, PEEP, P/F ratio, plateau pressure, and tidal volume. Traditionally with acute respiratory distress syndrome one would expect a decrease in compliance and the management is often plagued by difficulty in maintaining a plateau pressure <30 cm H2O. Interestingly we found no significant difference in compliance or plateau pressure between the cases and controls. We did however find a small but statistically significant change in PEEP between the two groups with the cases (intervention group) having a steeper trend towards lower PEEP (Table 3). The validity of this finding is supported by a statistically significant difference in applied FiO2 as well. This suggests that the cases had a statistically higher oxygen requirement, albeit marginal and not clinically significant. The apparent lack of clinical benefit is consistent with previously cited research indicating either no clinical benefit of CPT and/or only trends towards benefit if given earlier in the disease course. However, the physiologic changes suggest some effect of the intervention. Other findings included an increase in both diastolic and systolic blood pressure, although also a small change. We suggest that this may be related to the volume of colloid administered (~ 200ml), or perhaps some other hypertensive effect of CPT at the arterial cellular level.29,30
Strengths of this study include the advanced statistical methods and matched case-control design to adjust for confounding. Controls were successfully matched 3:1 to cases using age, sex, widely accepted COVID mortality risk factors. The control group was shown to have no significant difference in disease severity based on APACHE IV scores. We also were able to analyze a very larger number of data points over time (e.g., 857 separate measurements of static compliance, 6,316 measurements of PEEP, etc). Our total sample size is small (N = 47) and we were unable to perform secondary analyses on mortality as a consequence. Another limitation is that some ventilator measurements such as static compliance needed to be obtained and recorded by a respiratory therapist. Since this is not automated, some physiologic datapoints were acquired less frequently than other datapoints.
Ultimately CPT has fallen out of favor with the development and use of monoclonal antibodies and other therapeutics. However, with the rise of new SARS-CoV-2 variants such as BA.4 and BA.5, there are concerns for decreased effectiveness of monoclonal antibodies.31 Additionally, monoclonal antibodies are currently not approved for use in the inpatient, critically ill, population.32 CPT has the advantage of not requiring months to years of research and development prior to broad accessibility. Theoretically it will update the antibodies present as the hosts that it is derived from recover from new variants. Therefore, CPT could be utilized again in the future as new viral variants evade our current therapeutics.
Our study successfully evaluated basic physiologic parameters associated with the use of CPT in the critical care setting. Although we were able to find some statistically significant effects, we felt they were clinically insignificant and therefore consistent with the prior body of research including clinical trials suggesting no clinical benefit of CPT with this population.
DISCLOSURES/CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING INFORMATION
None to disclose
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ernest K. DiNino, MD
Department of Pulmonary and Critical Care Medicine
University of Massachusetts Chan Medical School-Baystate
3300 Main St Ste 2B, Springfield, MA 01107, United States. Email: ernest.dininomd@baystatehealth.org
Phone: (413) 794-7330
ORCID: 0000-0003-2465-655X