Introduction
Peritoneal dialysis (PD) is underutilized in the United States as a modality of kidney replacement therapy.1,2 There has been recent renewed interest in PD with novel reimbursement models supporting increased use, as well as emerging evidence that PD allows for comparable outcomes to hemodialysis (HD) with superior patient autonomy and at a lower cost.3,4 Hydrothorax is a rarely encountered but potentially lethal complication of peritoneal dialysis, which currently lacks clearly defined gold standards for diagnosis and treatment, leading to divergent approaches from providers. The typical presentation is thought to be one of dyspnea and pleurisy. We report an atypical presentation of a rare pathology, a case of hydrothorax that initially presented as fatigue and syncope. We also offer insights on possible etiological causes, methods of recognition, and management of this condition.
Clinical Presentation
A 54-year-old female with end-stage kidney disease on continuous cycling peritoneal dialysis for four months secondary to membranous glomerulopathy presented with a pubic ramus fracture after a syncopal episode resulting in a fall. Her recent history was notable for worsening fatigue, poor appetite, and difficult-to-control moderate hypertension. She had previously been counseled regarding options for dialysis and expressed a desire to pursue PD. Her PD prescription was four 1.8L exchanges over 8 hours without a day dwell, which recently was increased from 1.6L exchanges. She denied chest pain, dyspnea, or edema. Past medical history was also significant for secondary hyperparathyroidism, hyperphosphatemia, hypertension, iron-deficiency anemia, obstructive sleep apnea, and depression. She had a peritoneal catheter exit site leak during the initial flush, which resolved after rest. Medications included amlodipine, losartan, torsemide, metolazone, carvedilol, calcitriol, sevelamer, darbepoetin, venlafaxine, and omeprazole. She had no known drug allergies.
On examination, blood pressure was 98/64mm Hg, heart rate 78 beats/minute, respiratory rate 18 breaths/minute, and oxygen saturation 98% on ambient air. The cardiopulmonary exam was notable for decreased right-sided breath sounds. The mucous membranes were moist, and edema was absent. The peritoneal catheter exit site did not show drainage or erythema. Laboratory data on presentation labs included serum sodium 137 mEq/L (135-145), potassium 3.7 mEq/L (3.5-5.5), chloride 98 mEq/L (96-106), CO2 20 mEq/L (35-45), albumin 3.7 g/dL (3.4-5.4), blood urea nitrogen 64 mg/dL (6-24), creatinine 6.05 mg/dL (0.6-1.1), calcium 9.5 mg/dL (8.5-10.2), hemoglobin 9.1 g/dL (12-15), and WBC 5.1x10^9/L (4.5-11); prior relevant dialysis laboratory data included the peritoneal equilibrium test: Dialysate (4 hour)/Dialysate(0 hour) glucose 0.19, 4-hour Dialysate/Plasma creatinine 0.89 consistent with a high transporter; Dialysis adequacy - Total Kt/V 2.60 (>1.7), Dialysate Kt/V 1.82, Renal Kt/V 0.78, and urine volume 800 mL.
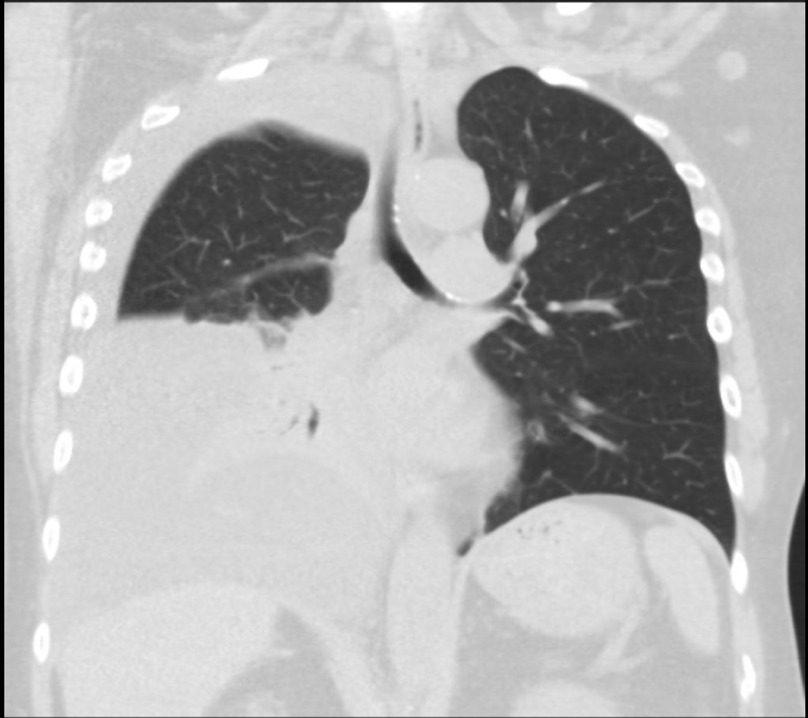
Imaging during trauma evaluation incidentally revealed a large right-sided pleural effusion with the collapse of the right mid and lower lung (Figure 1). Thoracentesis was performed with pleural fluid notable for pH 7.5, elevated glucose of 290 mg/dL, and undetectable protein and LDH, indicating a transudative effusion by Light’s criteria (Table 1). Serum glucose at the time was 128 mg/dL. A pleuroperitoneal leak was diagnosed. Peritoneal dialysis was continued with low volume exchanges with improvement in symptoms of fatigue and poor appetite as well as improved blood pressure control; however, the leak persisted after two months, and transition to HD was performed with a plan for future attempts to return to PD.
Discussion
Hydrothorax is a rare complication of peritoneal dialysis that typically presents with dyspnea, pleuritic chest pain, dullness to percussion, and hypoxemia. This patient presented without such expected features of hydrothorax but was diagnosed based on incidental findings consistent with pleural effusion on the chest radiograph and subsequent thoracentesis. Some authors have reported that as many as 25% of hydrothorax patients may be asymptomatic, but the exact proportion of PD-associated hydrothorax cases that present asymptomatically is unclear.1,2
The incidence of pleural effusions in the PD patient population is believed to range from 1.6% to 10%, with cases occurring more commonly in women than men.3,4 The underlying etiology of PD-associated hydrothorax has not been fully elaborated, but high intra-abdominal hydrostatic pressure from large dialysate volumes likely leads to transudative leak of dialysate fluid, often through a pleuroperitoneal communication.5,6 The pathophysiology of hydrothorax in peritoneal dialysis patients is commonly linked to congenital communications, diaphragmatic hernias, and fistulas, or acquired defects of subdiaphragmatic lymphatics, which serve as channels for the pleuroperitoneal leak.7 Hydrothorax dependent on diaphragmatic defects, is usually expected to appear within one month of initiating PD therapy and predominantly occur on the right side.6,8 In a study of 50 patients with PD-related effusions, 88% of cases were right-sided, likely due to the protective effects of the heart’s location on the left diaphragm.7 This case demonstrates an atypical presentation of an unusual complication, as the patient’s hydrothorax presented with non-specific symptoms and was delayed, occurring several months after her PD therapy start date.
Alternatively, hypervolemia as a sequela of poor ultrafiltration or heart failure can also lead to transudative effusions.9 Patients whose membranes exhibit fast solute transport rates can equilibrate solute and clear uremic toxins more rapidly, but also readily absorb dialysate dextrose, diminishing the blood-dialysate osmotic gradient and thereby weakening ultrafiltration pressure.10 Thus, poor ultrafiltration associated with this patient’s rapid transport peritoneal membrane characteristics in the setting of a diaphragmatic patency may have contributed to her presentation. Higher dextrose dialysate solutions are typically used to increase ultrafiltration to address respiratory symptoms. The resultant increased intra-abdominal fluid volume and pressure may paradoxically worsen pleural fluid accumulation. However, the high pleural fluid glucose is diagnostic of pleural leak rather than volume overload as the primary cause of her hydrothorax.
Rarely, underdialysis and consequent uremia have been shown to exacerbate or cause pleural effusions.11 Uremic pleuritis has previously been well-described, in which inflammation and fibrosis of the serosal lining along with loss of platelet function promote hemorrhagic transudation of fluid into the pleural space.12 However, as uremic pleuritis is an inflammatory process, it usually manifests as exudative effusion, in contrast with the transudative nature of this patient’s effusion.13
As PD use in the United States continues to rise, recognition of less common presentations is essential for early detection of PD-related complications, and for effective intervention strategies. As witnessed in this case, PD-related hydrothorax can often go undetected and be discovered only incidentally. The possibility for patients to present asymptomatically or atypically underscores the need for more immediate and precise modes of evaluating this pathology. Our threshold for suspicion of a pleural leak should be low even with nonspecific presentations, necessitating a thorough lung examination during monthly dialysis visits and imaging as indicated. While our patient’s pulmonary exam was unremarkable during the dialysis clinic visit preceding her syncopal episode, she had already reported fatigue. Implementation of point-of-care ultrasound during routine outpatient visits for patients on PD may facilitate early detection of pleural effusions, with superior sensitivity and specificity to physical exam alone shown in a non-dialysis population.14
A dramatic rise in pleural fluid glucose concentration relative to serum, which was appreciated in this patient, is caused by leakage of PD dextrose solution into the pleural space and is thus generally considered to be indicative of hydrothorax.15 Although a pleural glucose concentration >16.5 mmol/L has previously been suggested as a diagnostic cutoff, no definitive diagnostic standard exists, and this metric’s reliability is uncertain.8 It has been proposed that a significant difference of >50 mg/dL between pleural and serum glucose has close to 100% specificity. However, sensitivity remains low, and patients rarely present with such drastic findings.16 Another diagnostic method involves peritoneal scintigraphy with technetium 99m to localize and visualize the pleuroperitoneal defect, which has a sensitivity approaching 50% but is often more costly and time-consuming.17 CT peritoneography has also been used to diagnose and locate diaphragmatic communications, but has a sensitivity of approximately 33%, with CT radiocontrast dyes also posing potential risks of nephrotoxicity in patients with diminished renal capacity.18,19 MRI peritoneography avoids the pitfalls pertaining to toxic or radiation exposures, but is generally more expensive and less accessible than CT and other modalities.20
Initial management generally includes peritoneal resting along with peritoneal drainage or thoracentesis, after which sclerosing therapies can be attempted.3 Some advocate a trial of lower dialysate volumes, intermittent PD, or avoidance of overnight dwells.21 The typical course of management involves withholding PD with transition to HD to allow for a closure of the peritoneal defect.7 The ideal duration for the rest period is unknown. A number of cases have shown that reinstating treatment with low-volume PD is possible after undergoing two to six weeks of peritoneal resting.21 Nearly 60% of patients successfully resume PD following spontaneous healing of the peritoneal mesothelium.21 If hydrothorax recurs, fluoroscopic or surgical sclerosis of the pleuroperitoneal barrier prior to restarting PD is recommended.6 Direct obliteration of the diaphragmatic valve through video-assisted thoracoscopic surgery or open surgery has been used previously, but in some cases is unsuccessful given the potentially microscopic size of the defect.17 The overall success rate of thoracoscopic surgery is reported to approach 70%, with superior outcomes once the size and location of the defect is confirmed.19
Chemical pleurodesis with talc or tetracyclines has also been utilized; talc poudrage in particular, has demonstrated high efficacy, as nearly 90% of patients do not experience hydrothorax reaccumulation within 18 months of the procedure.17
Clinicians encountering peritoneal dialysis patients with pleural effusion should also be cognizant of non-dialysis-related etiologies of pleural effusion, which include heart failure, malignancy, parapneumonic effusion, tuberculosis, cirrhosis, and mesothelioma. Assessment of pleural fluid pH can assist in pinpointing the causes of exudative effusion; for instance, pH <7.3 is suggestive of an infectious or malignant etiology.22 In the case of our patient, despite peritoneal rest, she had a recurrence of pleural effusion upon resuming PD and was given imminently planned living donor kidney transplant; she proceeded to continue dialysis treatment with HD instead. Recent approaches have also included PD prescription modification with avoidance of overnight dwells, along with continuous pleural effusion drainage +/- pleurodesis, allowing patients to continue PD without reaccumulation.23
Conclusion
Hydrothorax is an uncommon but significant complication of PD requiring urgent detection and appropriate treatment. Standards of care for diagnosing and managing this complication have yet to be established. Our patient’s case highlights the difficulties in achieving rapid detection and long-term management of this condition, given her nonspecific initial presentation and tedious treatment course. Future research should focus on elucidating potential risk factors, early detection, and improving the sensitivity of diagnostic tools, which are imperative to patient survival. Furthermore, it is essential to note that in isolated hydrothorax cases, long-term PD treatment can be resumed and maintained once the condition is resolved.24
Acknowledgements
None.
Disclosures/ Conflicts of Interest
Ankur Shah reports consulting fees from Otsuka and CareDx.
Funding Information
The authors received no external funding for this work.
Author Contribution
All Authors have reviewed the final manuscript prior to submission.
All the authors have contributed significantly to the manuscript, per the ICJME criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Corresponding author
Ankur Shah
Division of Kidney Disease and Hypertension,
Rhode Island Hospital, Warren Alpert Medical School of Brown University,
375 Wampanoag Trail, East Providence, RI 02915.
(P) 401-649-4060 (F) 401-649-4061
Email: ashah8@lifespan.org