Background
Immune checkpoint inhibitors (ICIs) have revolutionized the field of medical oncology and have been shown to increase survival in many different tumor types.1 ICIs are monoclonal antibodies that block molecules responsible for inhibiting the immune response to cancer. This allows for T-cell activation, tumor recognition and immune-mediated destruction of cancer cells. The two primary groups include: inhibitors of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), a key inhibitory molecule involved in antigen presentation; and inhibitors of programmed cell death (PD-1) or its ligand, programmed cell death protein ligand 1 (PD-L1).2
A unique side-effect profile of ICIs is an overactivation of the immune system resulting in autoimmune destruction of otherwise healthy tissue, known as immune-related adverse events (irAEs). While rare, one irAE is the autoimmune destruction of renal parenchyma and the development of acute interstitial nephritis (AIN).2 Here, we present a patient with metastatic bladder cancer treated with pembrolizumab (an inhibitor of PD-1) who developed AIN, refractory to glucocorticoid therapy and salvaged by mycophenolate mofetil. In the context of clear metastatic disease and the development of multiple irAEs, patient had a complete response to his immunotherapy and remains in complete radiographic response more than 12 months later.
Case Presentation
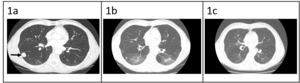
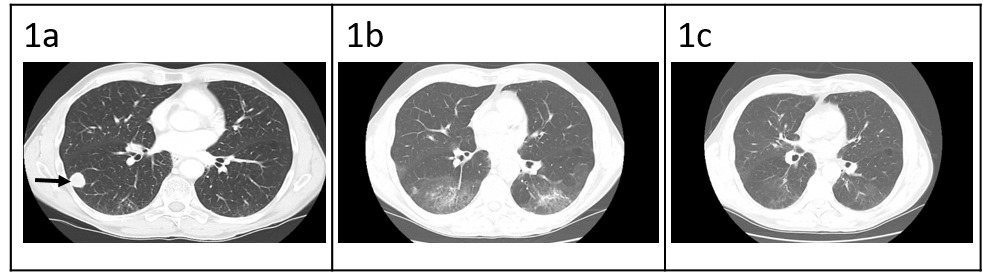
Patient is a 68-year-old male with prior history of nicotine dependence, chronic kidney disease (previous baseline creatinine ~1.3 to 1.4 mg/dL), and muscle invasive bladder cancer. He was initially diagnosed with Stage IIIA (cT3, cN0, cM0) disease3 in October 2019 and completed four cycles of neoadjuvant chemotherapy with gemcitabine and cisplatin followed by cystoprostatectomy.3 At time of surgical resection, he had persistent T3 disease. One year later, he was found to have metastatic recurrence to the lungs, right hilar nodes, and perineum (Figure 1a). The National Comprehensive Cancer Network guidelines for first-line therapy of metastatic bladder cancer is determined by cisplatin eligibility.3 In this patient with a prior poor response to neoadjuvant cisplatin-based chemotherapy, pembrolizumab monotherapy was chosen as front-line treatment for metastatic disease. After 10 cycles, he was incidentally found to have grade 1 pneumonitis on routine CT scan (Figure 1b). Pembrolizumab was held, and he was treated with a 1-month course of prednisone 1 mg/kg. Repeat CT chest after completion of steroids showed improvement of pneumonitis and a near complete radiographic response of his cancer (Figure 1c). He was not re-challenged with further pembrolizumab.
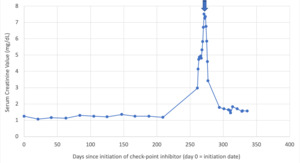
Two months after his last dose of pembrolizumab, the patient presented to the hospital with fever and dyspnea. His lab work-up was notable for serum creatinine elevated to 2.97 mg/dL from baseline 1.3 mg/dL. Chest and abdominal CT scan showed new multifocal patchy airspace disease concerning for recurrent drug-induced pneumonitis versus infection. He was found to be COVID-19 positive with an unrevealing bronchoscopy, and his respiratory symptoms improved with antibiotics alone. His renal function continued to worsen, and serum creatinine peaked at 7.51 mg/dL over a span of 10 days (Figure 4). Microscopic visualization of patient’s urine sediment showed renal tubular epithelial (RTE) cells as well as RTE casts. The leading differential was acute kidney injury due to acute tubular necrosis (ATN) in setting of multifactorial pre-renal etiology (volume depletion versus NSAID-use versus infection). However, given progressive renal decline and concern for an autoimmune process, patient ultimately underwent a renal biopsy that revealed interstitial inflammation, tubular necrosis, and glomerulosclerosis, confirmatory for ICI-induced AIN (Figure 2).
Patient was initially treated with IV methylprednisolone 500mg alone for two days without improvement in renal function. Given concern for steroid refractory AIN and impending dialysis needs, second-line mycophenolate mofetil (MMF) was added at a dose of 1000mg twice daily. After initiation of MMF therapy, patient’s renal function rapidly improved over 3-4 days (Figure 3). He was then discharged on prednisone 1mg/kg and mycophenolate mofetil 1000mg twice daily.
Patient received oral MMF 1000mg twice daily for a total of 10 days in addition to a prolonged taper of prednisone over a period of 4 months with close monitoring of his kidney function. He had a near complete recovery of his renal function. Most notably, the patient remains in complete remission, with most recent CT scan showing no evidence of recurrent disease despite being off pembrolizumab for approximately 15 months.
Discussion
Immune-related adverse events are common autoimmune side-effects seen with ICIs. In instances where anti-CTLA-4 inhibitors are used in combination with anti-PD-1 inhibitors, grade 3/4 irAEs can occur in up to 50-60% of patients.4 When anti-PD-1/PD-L1 inhibitors are used as monotherapy, incidence of toxicity is lower, with grade 3/4 irAEs occurring in 20-30% of patients.4 Given the expanding role ICI’s play in medical oncology, the prevalence of these toxicities continues to increase. It is critical that providers understand the unique mechanism and adverse effect profile of ICI’s to ensure prompt recognition and treatment.
Acute interstitial nephritis from ICIs is relatively rare and, as highlighted in the case above, can be difficult to diagnose. Like other etiologies of AIN, patients have sub-nephrotic range proteinuria and pyuria, but most ICI-induced AIN cases lack eosinophilia, fevers, and rashes.5,6 The temporal heterogeneity and variable time course from ICI-exposure to onset of acute kidney injury (AKI) is also unique with a recent review showing a time course from exposure toxicity ranging from 21 to 245 days.7 These atypical characteristics of AIN due to ICI can complicate the diagnosis and lead to interruptions of immunosuppressive therapy. In the case presented above, there were several confounding factors that delayed a renal biopsy for definitive diagnosis.
The exact mechanism by which ICI therapy results in AIN is not entirely clear. The variable time course and delayed onset of AKI suggests a distinct mechanism from a traditional delayed hypersensitivity reaction seen in other types of drug-induced AIN. It is hypothesized that PD-L1 expression in the proximal tubules of the kidney is critical for immune tolerance from self-reactive T-cells.1,8,9 Exposure to PD-1/PD-L1 inhibitors disrupt this immune homeostasis resulting in T-cell mediated destruction of proximal tubular cells.1,8,9
Glucocorticoids remain the standard of treatment for ICI-induced AIN based on published guidelines.10 In a recent review, 9 of the 10 patients with ICI-induced AIN treated with glucocorticoids had complete or partial recovery of their renal function.5–7 In a multi-center analysis of 138 patients with ICI-associated AKI, 86% of whom were treated with steroids, a complete response was seen in 40% of the patients, partial recovery in 45%, and no recovery in 15% of the patients.2 It was also shown that a higher weight-based dose of prednisone correlated with a higher rate of complete response versus a partial response (median 0.77 mg/kg versus 0.66 mg/kg).2 Unfortunately, for the patient described in this case, we did not see a robust response to high doses steroids alone, likely due to diagnostic challenges causing a delay in introduction of immunosuppressive therapy. He was started on second-line mycophenolate and ultimately achieved near complete recovery of his renal function.
MMF is a prodrug of mycophenolic acid that inhibits synthesis of guanosine nucleotides. Notably, MMF targets type II Inhibitor of Inosine Monophosphate Dehydrogenase (IMPDH) that are more preferentially expressed in activated forms of T and B lymphocytes.11 MMF is used widely in treatment of autoimmune hepatitis (including in ICI-treated patients), and thereby clinicians have expanded the utility of MMF in treatment of other irAEs.11 Review of recent literature also demonstrated that AIN patient refractory to steroid-therapy who were treated with MMF had at least partial recovery of renal function.7 Literature also described utilization of MMF in cases where administration of high-dose steroids is contraindicated (i.e. newly diagnosed type I diabetics).11 In this patient’s treatment course, diagnostic complications delayed initiation of immunosuppressive agents, leading to concern that treatment with glucocorticoid therapy alone may not fully achieve suppression of immune-mediated toxicity. Hence, prompt treatment with an additional immunosuppressant, specifically MMF, was deemed necessary for further therapeutic management of the patient’s worsening renal function.
In the case above, the patient had multiple irAEs, including both nephritis and pneumonitis. Despite the discontinuation of pembrolizumab in a patient with metastatic disease, he remains in complete remission almost 36 months after diagnosis. There have been several retrospective reviews showing an association between increased survival and the development of irAEs.12 Moreover, it has been shown that patients with multiple irAEs have a significant longer overall survival (OS) than patients with one irAE.13 It is felt that the development of autoreactivity may be reflective of an effector T-cell response to the tumor, and in the process, T-cells become exposure and activated to antigens/epitopes that are shared by both the tumor and healthy tissue.1,9
It is important to acknowledge that the patient above had ICI-induced AIN despite being off pembrolizumab for over 2 months. There is a growing body of literature supporting the concept of delayed irAEs, immune toxicity that occurs months to years after discontinuation of ICIs.10 This is an entity that is poorly understood, underdiagnosed, and unreported as many clinical trials stop reporting adverse events after 90 days. These delayed irAEs are likely related to the long half-life of these drugs (pembrolizumab has a half-life of 22 days) and a fundamental, long-term change to the homeostasis of a patient’s immune system.
Overall, early recognition of ICI-AIN is warranted to ensure prompt treatment initiation. In cases of diagnostic uncertainty, a renal biopsy is an important tool to help guide the role of immunosuppressive therapy. Glucocorticoids show mostly favorable response rates, do not affect patient survival or time to treatment failure, and should be utilized promptly to treat ICI-induced AIN.14 There is a clear association between irAEs and treatment efficacy, which leads to greater patient progression free and overall survival. Unfortunately, there is very little prospective data to guide how to best rechallenge these patients with ICIs moving forward, which involves a risk-benefit analysis with the patient and a multidisciplinary approach involving the Oncology and Nephrology team.
Disclosures/Conflict of Interest
None of the authors have any disclosures nor conflict of interest
Funding Information
None of the authors have received any funding for the above
Author Contribution
All Authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the ICJME criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CORRESPONDING AUTHOR
Matthew Austin, MD
Yale University School of Medicine
Smilow Cancer Hospital Care Center
25 Wells St. 2nd floor Westerly, RI 02891
Phone: 475-301-3718
Email: matthew.austin@yale.edu
_and_pas_(b)_stained_sections_showing_intense_interstitial_and_tubular_intraepithel.png)
_and_pas_(b)_stained_sections_showing_intense_interstitial_and_tubular_intraepithel.png)
