Background
G6PD deficiency is the most prevalent inherited erythrocyte disorder, affecting approximately 360 million people worldwide. G6PD deficiency is an X-linked enzyme deficiency disorder encompassing numerous allelic variants causing dysfunction of the G6PD enzyme. While this disease is classically considered to impact populations in tropical Africa and Asia, estimates show it impacts approximately 10% of African American men. The disorder is defined as reduced glucose-6-phosphate dehydrogenase activity. The G6PD enzyme functions to protect red blood cells against oxidative stressors.1 G6PD initiates the first step in the pentose phosphate pathway, reducing NADP into NADPH. Glutathione reductase requires NADPH to reduce glutathione; reduced glutathione detoxifies reactive oxygen species such as hydrogen peroxide, preventing them from causing oxidative stress (Figure 1).2 Erythrocytes are particularly vulnerable to oxidative stressors due to their role in oxygen transport, thus acute hemolytic anemia is the main associated pathology.2 G6PD deficiency can be diagnosed with a blood test to determine G6PD enzyme activity. In patients with acute hemolytic anemia, G6PD enzyme activity may be falsely normal due to mainly younger, less vulnerable red blood cells being present. Thus, enzyme activity testing should occur two to three months after an acute episode to provide an accurate indication of the patients’ enzyme activity.3
Rasburicase, a recombinant form of the enzyme urate oxidase, is a drug formulated to treat hyperuricemia, which can be caused by tumor lysis syndrome (TLS). TLS is an oncologic emergency caused by massive cell lysis, leading to significant electrolyte abnormalities, including hyperuricemia and hyperkalemia, and potentially fatal cardiac arrhythmias.4 TLS can occur following the administration of chemotherapy or spontaneously in cancer types with high proliferative rates or large tumor burden.4 Rasburicase rapidly reduces hyperuricemia by converting nephrotoxic uric acid into allantoin, which is more easily and safely cleared by the kidneys. However, this conversion creates hydrogen peroxide, a reactive oxygen species, as a byproduct.5 Consequently, rasburicase is contraindicated in patients with G6PD deficiency, as these patients are unable to detoxify the hydrogen peroxide produced in the conversion of uric acid to allantoin.6 While it is recommended that patients be tested for G6PD deficiency prior to starting rasburicase, this is not always possible due to the high risk of morbidity and mortality associated with TLS.
Case Presentation
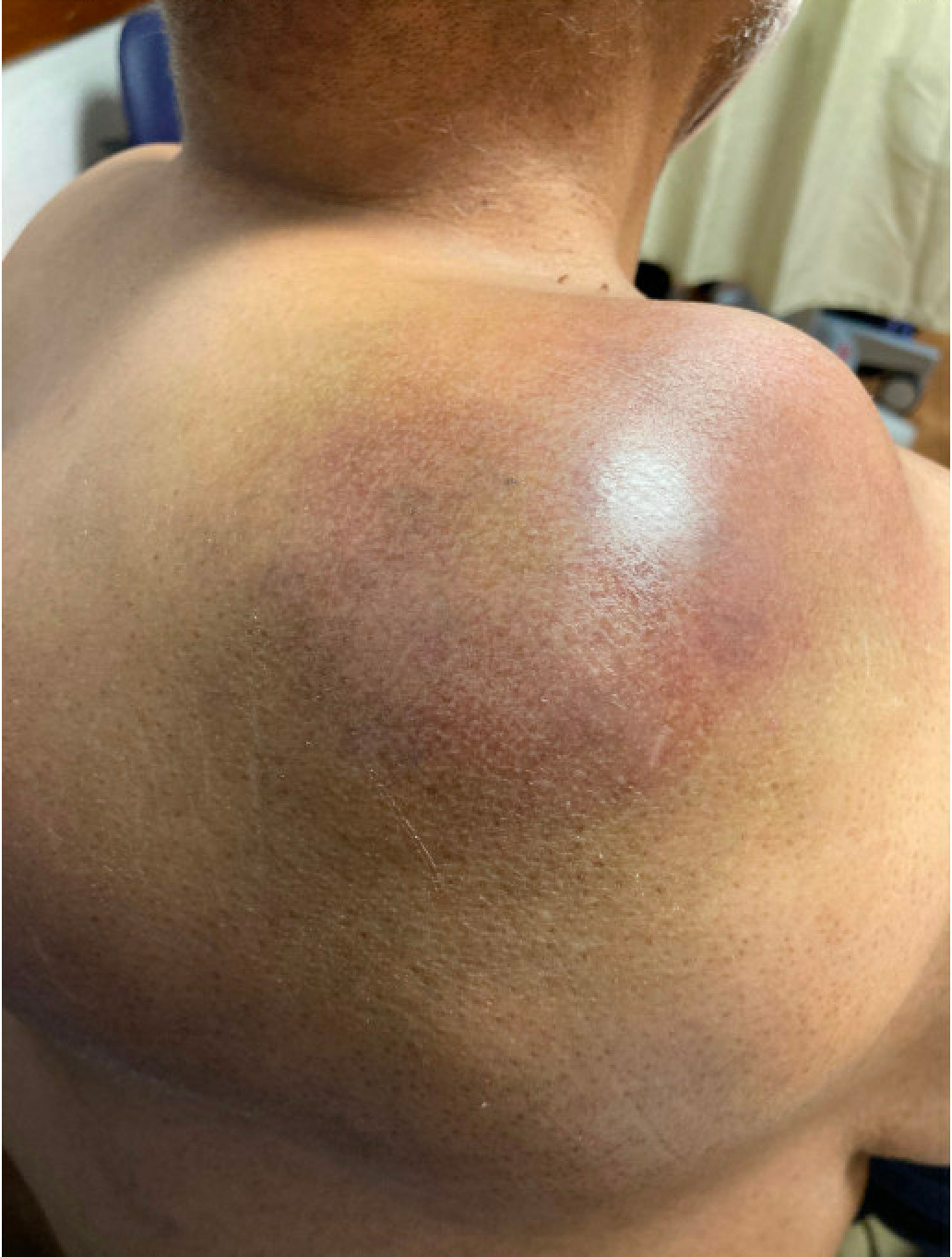
A 55-year-old African American male with a past medical history of diffuse large B-cell lymphoma (DLBCL) undergoing active chemotherapy, hypertension, and rheumatoid arthritis presented for fatigue and dark red urine. His home medications included losartan and his family history included Alzheimer’s disease in his mother. The patient was diagnosed with DLBCL approximately six months prior. Chemotherapy had thus far consisted of six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, which were discontinued due to disease progression, and one cycle of rituximab and polatuzumab vedotin. Previously, the patient was seen at his oncology clinic for evidence of cancer progression on his current chemotherapy, rituximab and polatuzumab vedotin, due to an increase in the growth of a bulky tumor on the patients’ back (Figure 2) The patient was urgently started on a new chemotherapy regimen consisting of ifosfamide, carboplatin and etoposide. Labwork at that time was notable for an elevated uric acid of 10.6mg/dl (reference range: 3.5 - 8.5 mg/dl.) Out of concern for TLS precipitating hyperuricemia in the setting of a high-grade tumor in addition to starting a new chemotherapy regimen, the patient was additionally given 10mg of rasburicase and started on 300mg of allopurinol daily. Five days later, the patient returned to his oncology clinic with fatigue and dark red urine.
Lab work revealed acutely worsened anemia and the patient was subsequently transferred to the hospital for blood transfusion and closer monitoring. Vitals on arrival, including oxygen saturation levels, were within normal limits, and his physical exam was notable for diffuse bulky tumors. Notable lab results included a hemoglobin of 5.9 g/dl (reference range: 13.5 - 16.0 g/dl) down from 12.4 g/dl five days prior, lactate dehydrogenase 2,511 IU/L (reference range: 100 - 220 IU/L), haptoglobin 13 mg/dl (reference range: 14 - 258 mg/dl), and reticulocyte count 367 cells × 109/L (reference range: 25 - 100 x109/L.) Blood smear was notable for schistocytes; Heinz bodies or bite cells were not seen. The patient’s acute anemia coupled with hemolytic markers such as elevated lactate dehydrogenase suggested hemolytic anemia. Direct antiglobulin testing was negative, characterizing the hemolysis as non-immune. Etiology of hemolysis was suspected to be undiagnosed G6PD deficiency in the setting of rasburicase exposure. The patient received a total of 3 units of packed red blood cells. The patient’s hemoglobin improved with red blood cell transfusion and cessation of rasburicase. Approximately three months after recovering from his acute anemia, the patient’s glucose-6-phosphate dehydrogenase level was tested and was 5.2 (reference range: 9.9 - 16.6 U/g Hb.)
Discussion
This case exemplifies the importance of considering G6PD deficiency as an etiology of acute hemolytic anemia when administering medications known to increase oxidative stress, such as rasburicase. Patients typically present with an acute 3-4 g/dl drop in hemoglobin along with dark urine due to hemoglobinuria, elevated lactate dehydrogenase, and elevated reticulocyte count two to four days following an offending stressor. A peripheral blood smear may show red blood cells with Heinz bodies due to the accumulation of damaged hemoglobin and bite cells as a result of splenic macrophages.7 Increased oxidative stress is seen in patients experiencing infection, diabetic ketoacidosis, or with recent exposure to medications or foods such as rasburicase, antimalarials, fluoroquinolones, sulfonylureas, or fava beans.8,9 The treatment of hemolytic anemia due to oxidative stress in patients with G6PD deficiency consists of supportive care such as stopping the offending stressor, treating the underlying cause, such as in cases of diabetic ketoacidosis, in addition to supplemental oxygen and blood transfusions.7 Ascorbic acid may also be used as it can detoxify reactive oxygen species without requiring NADPH.4 While steroids are effective in autoimmune hemolytic anemia, they are ineffective in non-immune mediated hemolytic anemia.7 In severe cases, patients may develop acute renal failure requiring hemodialysis.
When G6PD deficiency is suspected as an etiology for acute hemolytic anemia, the patient should also be monitored for signs of methemoglobinemia such as hypoxia and cyanosis. Methemoglobinemia, or an elevation of oxidized hemoglobin called methemoglobin, can also develop in patients with G6PD deficiency following an increase in oxidative stress. Since G6PD deficiency causes a lack of NADPH, the body cannot reduce methemoglobin to hemoglobin. Methemoglobin cannot bind oxygen to deliver it to the body, thus methemoglobinemia leads to tissue hypoxia.10 Symptoms to suggest methemoglobinemia include an asymptomatic hypoxia as read by a pulse oximeter, cyanosis, weakness, headache, fatigue, and chocolate colored blood.11,12 Methemoglobinemia classically presents with cyanosis, or bluish discoloration of the skin, particularly in the lips and nail beds.13 Importantly, pulse oximetry provides incorrect readings in the presence of significant methemoglobinemia because pulse oximeters measure the relative difference in wavelength absorption by oxyhemoglobin versus deoxyhemoglobin, however methemoglobin will absorb both wavelengths equally.11 In this case, the patient did not experience hypoxia and methemoglobin levels were not tested. While G6PD deficiency induced hemolytic anemia can cause hypoxia due to nonfunctioning, hemolyzed erythrocytes, it is important to also monitor the patient for methemoglobinemia.
Our patient was an African American male patient with acute symptomatic hemolytic anemia after receiving rasburicase, a known trigger of hemolytic anemia in patients with G6PD deficiency, which was resolved with supportive care. While the G6PD gene has nearly 400 known allelic variants, not all variants will result in a deficiency in the G6PD enzyme leading to hemolytic episodes. The variants that cause G6PD deficiency are grouped into classes correlating with disease severity, with class I being most severe and class IV indicating no disease. The two most common variants of G6PD deficiency are G6PD A- and G6PD Mediterranean. The G6PD A- variant is a Class III variant and is more common in people of African descent. The presentation of this patient as well as his ethnic background is consistent with the G6PD A- variant. The G6PD Mediterranean variant is a Class II variant, thus more severe, and typically affects Italian, Grecian, Spanish, Arabic, and certain Kurdish populations.14
Despite recommendations that high-risk patients such as African Americans and males are screened for G6PD status before starting rasburicase, screening is not always completed. In a 2016 study of G6PD testing prior to the administration of rasburicase in a large healthcare system, only 20.3% of patients identified as high risk for G6PD deficiency were tested.15 While rasburicase use is contraindicated in patients with G6PD deficiency, the acute risk of morbidity and mortality associated with untreated TLS creates a clinical dilemma. In patients without G6PD screening, waiting for the results of a G6PD activity screening test would delay TLS treatment, risking acute kidney injury and potentially fatal cardiac dysrhythmias.7 In these emergency situations, a single low dose of rasburicase (0.02 to 0.05mg/kg up to 3mg) can be administered and it is recommended that hemodialysis be readily available in the event of clinically significant hemolysis and acute renal failure.16 While the ideal dose of rasburicase for all patients with TLS is suggested to be a single dose of 6mg, studies indicate that a 3mg dose may be adequate for most cases of hyperuricemia.17 It is unclear whether this decreased dose of rasburicase (≤3 mg) mitigates hemolysis in patients with G6PD deficiency.7 As the extent of hemolysis is linked to both the level of G6PD deficiency and the dose of the offending drug, cautious use of rasburicase is recommended.
This case highlights the importance of precautionary G6PD deficiency testing in patients that are at high risk for TLS and may receive rasburicase. Knowing the patient’s G6PD status is particularly important if the patient is in a high-risk group, as our patient was due to his African heritage. In situations where G6PD testing is not feasible due to the urgency of requiring TLS treatment, clinicians should closely monitor patients for hemolytic anemia and methemoglobinemia. Recognizing high risk populations for G6PD deficiency as well as the manifestations of G6PD deficiency is critically important in preventing adverse events in oncologic care.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
• Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
• Drafting the work or revising it critically for important intellectual content; AND
• Final approval of the version to be published; AND
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/ Conflicts of Interest
The authors have no conflicts of interest to disclose.
CORRESPONDING AUTHOR:
Olivia Avidan MD
Department of Internal Medicine
Warren Alpert Medical School of Brown University
Rhode Island Hospital
Providence, RI 02903
Email: oavidan13@gmail.com