Background
Cocaine, a systemic toxin, is known to cause a wide range of effects throughout the body. Within the kidneys, the most described mechanism of injury is rhabdomyolysis followed by tubular damage. While acute tubular injury (ATI) and acute interstitial nephritis (AIN) have been associated with cocaine use in previous studies, the coexistence of these pathologies has not been previously reported.1 This report presents a rare case involving simultaneous tubular and interstitial involvement in the absence of rhabdomyolysis.[^1]
Case Presentation
A 37-year-old male with no significant past medical history presented to the hospital with complaints of left flank, epigastric pain lasting for three days, and nausea and vomiting. The patient denied recent medication or supplement use but admitted to consuming alcohol daily, with no reported drug use. Upon initial examination, his heart rate was 80 bpm, blood pressure was 147/84 mmHg, oxygen saturation was 100% on room air, and he was afebrile. The patient appeared euvolemic, without skin rashes, petechiae, or purpura. Cardiac, chest, and abdominal examinations yielded normal findings.
Upon admission, laboratory tests revealed elevated levels of blood urea nitrogen (BUN) at 58 mg/dL (reference range: 6-20 mg/dL) and serum creatinine at 10.74 mg/dL (reference range: 0.40-1.30 mg/dL). Additionally, the patient exhibited a serum bicarbonate level of 19 mmol/L (reference range: 20-30 mmol/L), an anion gap of 23 (reference range: 7-17), and phosphorous levels of 9.6 mg/dL (reference range: 2.2-4.5 mg/dL). Hemoglobin, platelet count, and coagulation tests were within normal limits. Urinalysis showed 1+ proteinuria, 1 red blood cell per high-power field (RBC/HPF), and 1 white blood cell per high-power field (WBC/HPF). The protein/creatinine ratio was 0.57 mg/mg Cr (reference range: <=0.10 mg/mg Cr), and urine sodium was measured at 47 mmol/L. Urine toxicology screening yielded a positive result for cocaine. On further questioning, the patient admitted to inhaling cocaine approximately 14 hours prior to admission. He denied use of any other nephrotoxic drugs, including non-steroidal anti-inflammatory drugs (NSAIDs).
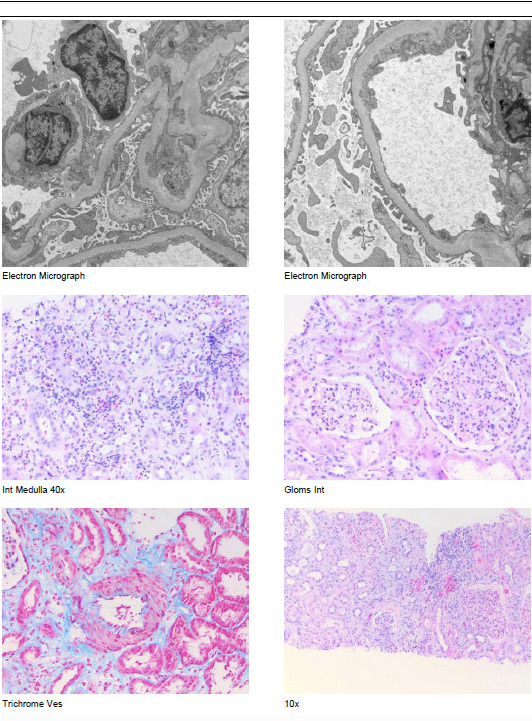
Further investigations revealed normal creatinine kinase levels (66 IU) and serum protein electrophoresis. Tests for hepatitis B, hepatitis C, and HIV were negative. C3 and C4 levels were within the normal range. The antinuclear antibody (ANA) test was negative, while the antineutrophil cytoplasmic antibody (ANCA) test showed a positive result at a titer of 1:40, indicating a perinuclear pattern. Renal ultrasound demonstrated normal-sized kidneys (right kidney: 12.1 cm, left kidney: 12.6 cm) without evidence of obstructing stones or hydronephrosis. Abdominal and pelvic CT scans performed without contrast revealed no significant abnormalities, and the chest X-ray showed no parenchymal or pleural pathologies. Given the persistently high creatinine levels of 10-11 mg/dL, the decision was made to proceed with a renal biopsy. The biopsy revealed focal acute tubular atrophy with sloughing of brush border and epithelial simplification, mild, diffuse interstitial edema, and patchy foci of active interstitial infiltrate suggestive of acute interstitial nephritis (Figure 1).
Conservative management was employed during the patient’s hospital stay, as his serum creatinine levels exhibited a downward trend. Throughout the hospitalization, the patient maintained a urine output of 800 ml to 1 L daily and did not require dialysis. Given the improvement in kidney function, corticosteroid therapy was not initiated. Spontaneous improvement in creatinine levels was observed in the subsequent days, with a reading of 2.91 mg/dL at discharge and 1.67 mg/dL at the first follow-up visit.
Discussion
Cocaine, a stimulant derived from the leaves of the Erythroxylon coca plant, is widely abused due to its euphoric properties. It is typically administered through various routes, including intranasal (snorting), oral (rubbing against the gums), intravenous (dissolving in water and injecting into veins), and inhalation (smoking). Less common routes of administration include vaginal and rectal administration. Among these routes, intravenous use and inhalation are associated with the highest dependence rates.2 We present the first case of renal failure secondary to biopsy-proven concomitant ATI and AIN in the setting of cocaine use.
Cocaine exerts its effects by acting as a potent vasoconstrictor and stimulating both the sympathetic and dopaminergic systems. It inhibits norepinephrine reuptake at the presynaptic vesicle of neurons, leading to increased levels of norepinephrine and epinephrine release from the adrenal medulla. Additionally, it impedes nitric oxide-mediated vasodilation. The vasoconstrictive properties of cocaine extend beyond the kidneys and can result in various extrarenal manifestations, including myocardial infarction, hypertensive crisis, stroke, and tissue ischemia.3
Cocaine has been reported to affect all compartments of the nephron within the kidneys. In the vascular compartment, it induces vasoconstriction, endothelial dysfunction, and platelet aggregation, which can manifest as infarction or thrombotic microangiopathy, particularly in cases of malignant hypertension. In the glomerulus, cocaine can contribute to a nephritic presentation in conditions such as immune complex glomerulonephritis (including ANCA-associated vasculitis associated with levamisole in cocaine use), post-infectious glomerulonephritis (resulting from bacterial infections, including skin infections, infective endocarditis, hepatitis C-associated MPGN, or rarely, mixed cryoglobulinemia associated with Hepatitis C infection- all associated with specific routes of cocaine use or contaminated ingredients). Conversely, a nephrotic syndrome-like presentation can be observed in focal segmental glomerulosclerosis (more commonly associated with heroin use in the black population), and AA amyloidosis (frequently observed in heroin users). Additionally, in the tubulointerstitium, acute tubular injury (with or without rhabdomyolysis) and acute interstitial nephritis are among the possible presentations. It is important to note that repeated insults to the kidneys, regardless of the cause, can ultimately lead to the development of chronic kidney disease.1 Figure 2 provides a graphical representation of the spectrum of cocaine-induced acute kidney injury (AKI) and its various clinical manifestations.
Among the various manifestations, rhabdomyolysis-induced acute tubular necrosis (ATN) is the most common cause of AKI in cases of cocaine abuse. The pathogenesis is thought to involve the direct toxicity of cocaine, which leads to muscle degeneration and the release of heme and myoglobin pigments. These substances can cause tubular obstruction and trigger the release reactive oxygen species. Furthermore, cocaine-induced intense vasoconstriction can significantly reduce blood flow to the renal medulla, potentially resulting in ischemic acute tubular injury.4
In this case, rhabdomyolysis was ruled out as the cause of acute kidney injury (AKI) due to the patient’s normal admission creatine kinase (CK) levels. Considering the half-life of serum CK is approximately 17 hours, it is unlikely that rhabdomyolysis developed prior to admission.5 Additionally, the clearance of CK is further delayed in the presence of renal failure. Therefore, the absence of elevated CK levels at admission, along with the patient’s last cocaine use being 14 hours prior, effectively ruled out rhabdomyolysis as the underlying cause of AKI. Furthermore, there were no findings of sterile pyuria, eosinophiluria, or granular casts, suggesting acute interstitial nephritis (AIN) or acute tubular injury (ATI) as the etiology, respectively. Renal ultrasound did not reveal evidence of postrenal causes, and the absence of improvement with fluid administration precluded a prerenal state.
Due to the limited diagnostic utility of non-invasive investigations, a kidney biopsy was performed, revealing ATI and AIN. There was no evidence of alternative etiologies of ATI, such as sepsis or documented hypotension. The relatively short duration and self-resolution of AKI in this case was attributed to cocaine-induced vasospasm. It is debatable whether a single exposure to a vasoactive agent only 14 hours prior to his admission without concomitant nephrotoxin exposure would lead to this level of renal involvement in a person with normal renal function at baseline. The accuracy of the history of timing and frequency of cocaine use is probably questionable.
The occurrence of ATI without evidence of rhabdomyolysis associated with cocaine use is rarely reported, and only a few prior cases have been described in the literature.6–9 Table 1 summarizes the key clinical features of each reported case. Like these cases, our patient experienced non-oliguric AKI and remained euvolemic during hospitalization. After cessation of cocaine, renal function improved within two weeks. Glucocorticoids were not administered. The course of AKI with spontaneous recovery within 7-10 days aligns with previously reported cases of cocaine-induced AKI without rhabdomyolysis, suggesting a reversible process with a favorable prognosis.
AIN resulting from cocaine use is not a common cause of AKI. The exact mechanism, whether it involves cocaine itself or contaminants, remains unclear. Our patient did not exhibit the classic triad of “fever, rash, and eosinophilia,” which is observed in only 10-30% of AIN cases, according to previous studies. Other common etiologies of AIN, including antibiotic use, Tubulointerstitial nephritis and uveitis (TINU) syndrome, and autoimmune entities like lupus, were ruled out clinically and through appropriate testing. Notably, the patient had a positive perinuclear anti-neutrophil cytoplasmic antibody (ANCA) titer. However, the absence of other features of ANCA-associated vasculitis and relatively low titer makes it irrelevant as a potential etiology. The association of ANCA-associated vasculitis with levamisole-adulterated cocaine use is well-documented. The exact mechanism is not known but postulated to be due to generation of autoantibodies including ANCA. Treatment is primarily supportive and needs cessation of further cocaine use.10 However, whether it can cause asymptomatic elevation in ANCA titers is not well-described. The temporal relationship between the patient’s crack cocaine use and the development of ATI and AIN suggests that cocaine is the responsible agent.
In conclusion, this report presents a case of cocaine-induced AIN with concurrent ATI without rhabdomyolysis. It emphasizes the importance of considering cocaine as a potential cause of acute kidney injury (AKI), mainly when other obvious etiologies are absent. In this case, the prognosis of cocaine-associated AIN and ATI was favorable, with no residual renal insufficiency. The absence of perfusion injury or concomitant nephrotoxins would be vital contributory factors in determining a good prognosis. The findings underscore the diagnostic value of kidney biopsy in confirming the diagnosis of AIN and highlight the need for further research to gain a comprehensive understanding of the clinical course and optimal treatment strategies for cocaine-associated AIN and ATI.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/ Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that might influence the data reported in this paper.
Acknowledgements
We acknowledge the contributions of all medical professionals involved in the care of this patient. We also acknowledge the kindness of the patient and her family who agreed to publish this case with the intent of disseminating medical knowledge.