Background
The presenting symptoms of pulmonary embolism (PE) are diverse and include dyspnea, pleuritic chest pain, syncope, cough, hemoptysis, and symptoms associated with deep vein thrombosis (DVT).1 Cough is an extremely common symptom with a broad differential diagnosis, but cough as an isolated symptom in the presentation of PE is rare.1,2 It has been suggested that increased pressure in the pulmonary vessels and right atrium may cause a sensation of dyspnea and trigger cough.1 Given cough is the most common respiratory symptom encountered by clinicians and the significant morbidity and mortality of undiagnosed PE, this presents a diagnostic challenge.2
Case Presentation
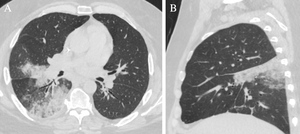
A man in his 50s with a history of heart failure with reduced ejection fraction, multiple venous thromboembolic events (VTEs), and bariatric surgery (sleeve gastrectomy) three years prior to presentation for morbid obesity presented with two weeks of cough. Although his cough was initially mild and dry, the frequency of cough intensified to one coughing fit per minute; he then developed hemoptysis (<1 tablespoon) and chills. He additionally had new dyspnea on exertion–which he felt was mostly related to uncontrolled bouts of cough–and severe “back” pain localized over the right scapula, which he described as “stabbing” pain made much worse by cough. Given he did not improve with a 3-day course of azithromycin, he presented to our hospital. Regarding his past medical history, he was noted to have had a first episode of DVT four years prior to presentation without a clear inciting factor. Work-up for Prothrombin and Factor V Leiden mutations, Protein C/S and Antithrombin activity derangements, and antiphospholipid antibody syndrome was negative. Two years prior to presentation, he had a second DVT during a hospitalization for pneumonia despite pharmacologic thromboprophylaxis, so he was started on lifelong rivaroxaban. Approximately six months prior to the current presentation, he underwent elective thrombolysis of the latter DVT, which was then considered chronic. Two weeks post-operatively, the patient underwent contrast-enhanced computerized tomography (CT) of the chest, which showed a non-occlusive PE within the right interlobar pulmonary artery (Figure 1). He was continued on rivaroxaban. Anti-Xa levels were never obtained.
On presentation, the patient was afebrile with a heart rate of 117 beats per minute, blood pressure of 166/112 mm Hg, and peripheral oxygen saturation of 96% on room air. Pulmonary exam was notable for right lower lobe crackles and moderate respiratory distress during frequent coughing episodes. Cardiac exam was notable for no murmurs and no jugular venous distension. Extremity exam showed 1+ bilateral pitting lower extremity edema to the mid-shin level. Laboratory studies were notable for a creatinine of 2.00 mg/dL (0.40 – 1.30 mg/dL) from a baseline of 1.50 mg/dL, BUN of 40 mg/dL (6 – 20 mg/dL), white blood cell count of 8,100 cells/µL (1,000 – 4,000 cells/µL), procalcitonin of <0.05 ng/mL (<0.25 ng/mL), high-sensitivity C-reactive protein of 15.8 mg/dL (<1.0 mg/dL), and aspartate aminotransferase of 13 U/L (11 – 204 U/L). The complete metabolic panel was otherwise normal. B-type natriuretic peptide was 6,483 pg/mL (< 125 pg/mL) with no baseline from prior, and high-sensitivity troponin was 38 ng/L (< 12 ng/L) and stable. All infectious studies were negative. A transthoracic echocardiogram showed a left ventricular ejection fraction of 20% decreased from 35%, severe left atrial enlargement, moderate right atrial enlargement, and no significant valvular abnormalities.
Chest radiography revealed a new right perihilar opacity (Figure 2). Axial images of a CT scan of the chest showed ground glass and consolidative opacities within the posterior superior aspect of the right middle lobe and superior segment of the right lower lobe (Figure 3a). Sagittal images showed wedge shaped airspace disease (Figure 3b). Given the patient was initially felt to have community-acquired pneumonia, he received 7 days of ceftriaxone and doxycycline, but he continued to have frequent cough and worsening “back” pain exacerbated by cough. Upon re-review of the patient’s initial chest CT (Figure 3), recognition of pulmonary infarction as a potential explanation for the patient’s symptoms led to acquisition of a contrast-enhanced chest CT, which revealed interval increase of the patient’s previously recognized PE within the right interlobar pulmonary artery, with new occlusion of the right lower lobe pulmonary artery and vessel expansion compatible with acute on chronic thrombosis (Figure 4a). The study additionally demonstrated progression of alveolar edema and hemorrhage within the right middle and right lower lobes (Figure 4b). Thrombectomy was considered; however, as there was no evidence of right heart strain or hemodynamic instability, this was deferred. Rivaroxaban was stopped, and a heparin drip was initiated with a 5-day bridge to warfarin. Warfarin was chosen over an alternative direct-acting oral anticoagulant (DOAC) due to concern for DOAC malabsorption related to the patient’s sleeve gastrectomy, as well as a desire for international normalized ratio (INR) monitoring.
On follow-up three months after discharge, the patient’s cough and “back” pain continued to improve, and his dyspnea on exertion had resolved. A follow-up contrast-enhanced chest CT showed persistent occlusion of the right lower lobe pulmonary artery with resolution of vessel expansion compatible with contraction of chronic thrombus (Figure 5a). Linear and wedge-shaped hyperdensities within the right middle and right lower lobes were consistent with scarring secondary to pulmonary infarction (Figure 5b).
Discussion
Pulmonary infarction as a complication of PE is typically diagnosed by the combination of characteristic chest imaging findings and suggestive clinical features (e.g., pleuritic pain, cough, hemoptysis, pleural friction rub).3 It occurs in an estimated 30% of patients with PE, although estimates range from 10 to 50% due to varying definitions of pulmonary infarction in the literature.3 Pulmonary infarction must be distinguished from alveolar hemorrhage, which occurs acutely after PEs and causes blood to extravasate into alveolar airspaces.3 Pulmonary infarction is established if alveolar hemorrhage progresses to necrosis and fibrosis.3 Since both alveolar hemorrhage and infarction lead to radiographic infiltrates, the diagnosis of infarction therefore requires follow-up imaging, after a patient’s pulmonary consolidation progresses out of the acute hemorrhagic stage.3 The risk of progression to true infarction appears to depend on pulmonary venous pressure: if pulmonary venous outflow is restricted, such as in heart failure, infarction is more likely to occur.4
While rounded, peripheral pulmonary infiltrates are considered classic for pulmonary infarction, the radiographic and pathologic shape of infarction is more often “randomly” shaped due to the structure and arrangement of the secondary pulmonary lobules.5 Since secondary pulmonary lobules are randomly organized polyhedra, a group of infarcted secondary pulmonary lobules may take on many shapes.5 Additionally, while most pulmonary infarctions extend to the lung periphery, autopsies have confirmed that infarctions need not contact the pleural surface.5 In fact, there are reports of non-peripherally located pulmonary infarctions mimicking lung neoplasm that have prompted unnecessary surgery.5
After the US Food and Drug Administration approved DOACs for the secondary prevention of VTE, DOACs quickly overtook warfarin as the most commonly initiated oral anticoagulants for VTE, and the American Society of Hematology currently recommends DOACs as the treatment of choice for VTE.6,7 While studies comparing VTE patients’ mortality and PE risk show DOACs do not provide an advantage over warfarin, DOACs do not require the INR monitoring, dietary restrictions, and dose adjustments required in warfarin use.7 Overall failure rates of anticoagulation with DOACs are low, with failure being more common in patients with a hypercoagulable condition such as malignancy and antiphospholipid syndrome as well as in patients with mechanical heart valves.8 However, DOAC failure due to gastrointestinal malabsorption related to bariatric surgery is increasingly recognized.9
Bariatric surgeries include adjustable gastric banding, Roux-en-Y gastric bypass, biliopancreatic diversion with duodenal switch, and sleeve gastrectomy. In general, bariatric surgeries can affect DOAC absorption by reducing caloric intake (in drugs that have increased absorption in the presence of food), decreasing absorptive surface area, changing the location of drug absorption, and altering how drugs transit through the intestinal tract.9 Of the DOACs, rivaroxaban has specifically been shown to be primarily absorbed in the proximal small intestine, to have some gastric absorption, and to have increased bioavailability when ingested with food.9 Apixaban has the same locations of absorption but is unaffected by concurrent food intake.9 Edoxaban is absorbed only in the proximal small intestine and unaffected by concurrent food intake.9 Finally, dabigatran is absorbed in the lower stomach and duodenum and unaffected by concurrent food intake.9 Despite these changes to absorption, retrospective studies of DOAC use after bariatric surgery have found relatively low rates of breakthrough clotting events (i.e., recurrent VTE and systemic embolism related to atrial fibrillation) of no more than 5.8%.10–12
While warfarin is thought to be absorbed primarily in the stomach and proximal small intestine, warfarin dose requirements initially decrease in patients in the early postoperative period after bariatric surgeries (i.e. less than three months post-operatively), with a subsequent increase in dose requirements after that.9,13 Given its relationship to vitamin K, such effects may be mediated by decreased vitamin K absorption, unstable dietary intake, and altered gastrointestinal tract function.13 Of note, there have been no randomized controlled trials comparing VTE outcomes in patients receiving warfarin versus DOACs after bariatric surgery.14 One meta-analysis assessing VTE risk after bariatric surgery demonstrated a higher pooled incidence of VTE with DOAC treatment compared to warfarin, but this analysis had several limitations.14
In conclusion, in patients who have a history of bariatric surgery and DOAC use for VTE, the development of recurrent VTE should prompt consideration of poor DOAC absorption. While commercial assays for serum DOAC levels are now available, reference ranges are variable and poorly correlated with clinical outcomes.9,15 In such patients, a transition in anticoagulation to warfarin may be considered for more reliable drug monitoring.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures & Conflicts of Interest
The authors declare no conflicts of interest.
Corresponding Author
Andrew Sanchez, MD
Yale University Department of Internal Medicine
333 Cedar Street, P.O. Box 208056
New Haven, CT, USA 06510
Phone: 203.688.9503
Email: andrew.sanchez@yale.edu