Background
Myocardial bridging occurs when a portion of the epicardial coronary artery tunnels underneath a band of myocardium. Myocardial bridging can be asymptomatic or cause a variety of complications including acute coronary syndrome, coronary vasospasm, ventricular septal rupture, and arrhythmias. The most commonly involved epicardial coronary artery is the left anterior descending usually the middle segment. Myocardial bridging has largely been considered a benign condition however symptomatic patients who are refractory to medical management often undergo surgical intervention with unroofing procedures and rarely coronary artery bypass graft surgery. This case presents a unique clinical conundrum for the treatment of a young patient with coronary artery bypass grafting to treat refractory chest pain symptoms despite pharmacological therapy due to significant myocardial ischemia. Long term consequences of early coronary artery bypass grafting are unknown in this population.
Case Description
A 27-year-old woman with history of hypertension, diabetes mellitus, hyperlipidemia, and family history of sudden cardiac death presents to the hospital with complaints of substernal chest pain and palpitations. She describes the chest pain as a “thunderclap of substernal sharp to pressure-like unrelenting pain that lasts minutes to hours associated with sweating, shortness of breath, and palpitations”. She first noted these symptoms started approximately 4 weeks prior to admission but have been occurring more frequently with the intensity and duration becoming significantly longer from a few minutes to a few hours. The most significant pain occurs when she wakes up from sleep. Physical examination was benign. Review of systems also positive for loss of appetite, anxiety, dizziness, nausea, and vomiting. Labs were significant for electrolytes abnormalities related to her nausea and vomiting (hypokalemia 3.2 mEq/L, normal 3.5 - 5.3 meq/L, hypocalcemia 6.7 mg/dL, normal 8.6 - 10.5 mg/dL, hypomagnesemia 1.5 mg/dL, normal 1.8 - 2.4 mg/dL), uncontrolled glucose 300 mg/dL (normal 70-100 mg/dL). Other lab results including pregnancy, troponin, urinalysis, thyroid stimulating hormone, Helicobacter pylori antigen, lipase was all within normal limits. Electrocardiogram demonstrated normal sinus rhythm. Vital signs within normal limits. Echocardiogram demonstrated normal ejection fraction of 66% without any significant valvular abnormalities.
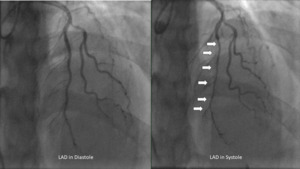
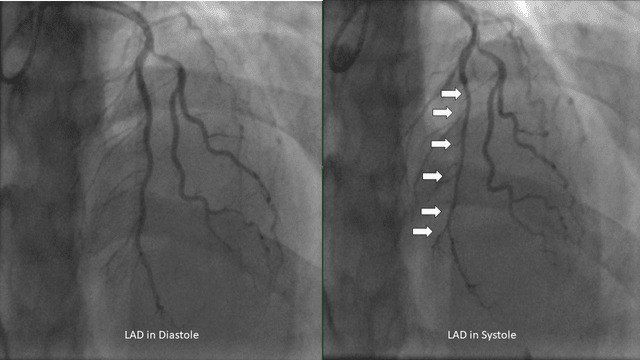
Given her age, she first underwent computed tomography coronary artery imaging which demonstrated a long intramuscular segment of the left anterior descending artery without significant stenosis, separate origin of the left anterior descending and left circumflex coronary arteries from the left coronary cusp without a left main coronary artery, high-grade stenosis of the origin of the 1st diagonal. She then underwent a coronary angiogram which revealed hemodynamically significant myocardial bridging of the left anterior descending (resting-full-cycle-ratio, RFR positive at 0.76) indicative of myocardial ischemia during systole, moderate lesions involving the 1st diagonal (60%) which was RFR negative at 0.93 and 1st/2nd obtuse marginals (30% and 50% respectively) (Figure 1). She was placed on medical therapy with beta-blockers and calcium channel blockers without significant improvement despite 4 weeks of therapy at the highest doses tolerated. A Heart Team approach involving the Interventional Cardiologist, Cardiology Consult Team, Cardiothoracic Surgery, and the patient ultimately resulted in the decision for bypass surgery given her constant, unrelenting symptoms of unstable angina.
Cardiothoracic surgery did not feel that an unroofing procedure would be adequate given the length of the intramuscular segment and therefore opted to do a 3-vessel coronary artery bypass. A midline sternotomy was used to access the chest cavity. The left and right internal mammary arteries were exposed using a skeletonized technique. The first diagonal was opened proximally, and the left internal mammary artery was anastomosed side-to-side, subsequently, the distal left anterior descending artery was sequentially grafted using an anastomosed side-to-side technique. The right internal mammary artery was redirected through the transverse sinus and anastomosed end-to-side to the 1st obtuse marginal artery. She had an uneventful post-operative course and had full relief of symptoms.
Discussion
Myocardial bridging is diagnosed by coronary angiography with systolic narrowing of the epicardial coronary artery.1–5 The prevalence of myocardial bridging occurs in approximately 1.3% of the general population.1–3 The most commonly involved epicardial coronary artery is the mid left anterior descending though in autopsy series the right coronary artery and the left circumflex artery appears to have similar rates of occurrence.5,6 There are two distinct types of myocardial bridging, superficial (approximately 75% of cases) and deep (25% of cases), of which hemodynamic compromise occurs more frequently in the deep variant.6 Symptomatic cases of myocardial bridging are often treated successfully with beta-blockers or calcium channel blockers.6,7 However, in refractory cases it has been proposed that percutaneous stent placement can prevent external compression however in limited studies the rate of restenosis or stent fracture remains too high to be recommended in symptomatic patients.1,6,7 Surgical intervention with unroofing or coronary artery bypass grafting surgery remains relatively safe and the treatment of choice for myocardial bridging.1,7,8
Extensive myocardial bridging refers to cases where the tunneled segment of the coronary artery extends over a considerable length (>2.5 cm, and >0.5 cm deep), resulting in significant compression and compromised blood flow.1,7,8This compression during systole can lead to myocardial ischemia and symptoms such as angina, dyspnea, or even myocardial infarction in severe cases.1,2 Coronary angiography remains the gold standard for visualizing systolic compression or “milking” of the coronary artery.4,6 Other methods of assessing systolic compression include intracoronary doppler, intravascular ultrasound, fractional flow reserve, and cardiac computed angiography.4–6
In mild to moderate cases of myocardial bridging, conservative management strategies are often employed.3,6,8 This includes pharmacological therapy with beta-blockers, calcium channel blockers, or ivabradine to alleviate symptoms and reduce myocardial oxygen demand.3,6–9 However, in cases of extensive myocardial bridging where conservative measures fail to provide adequate relief or when the symptoms are severe and debilitating, more aggressive intervention may be required.4,7 Coronary artery bypass grafting (CABG) has been utilized as a therapeutic option for extensive myocardial bridging, particularly in cases where the patient experiences persistent symptoms despite conservative management.2–4,7,9 CABG involves creating bypass grafts to restore blood flow beyond the bridged segment of the coronary artery, effectively bypassing the area of compression.1,2,4,6
Studies investigating the efficacy of CABG in extensive myocardial bridging have reported favorable outcomes in terms of symptom relief and improved myocardial perfusion.1,7 A significant reduction in angina frequency, improvement in exercise tolerance, and resolution of ischemic changes on stress tests have been observed post-CABG.3 Additionally, long-term studies have demonstrated sustained benefits with low rates of recurrent symptoms and re-intervention.2,7 Like any surgical procedure, CABG for myocardial bridging carries inherent risks. These include perioperative complications such as bleeding, infection, arrhythmias, or myocardial infarction.7,8 Additionally, long-term considerations such as graft patency and the potential for disease progression in non-bridged segments of the coronary arteries must be considered.3,7,8Extensive myocardial bridging can pose a significant clinical challenge, especially when conservative management fails to alleviate symptoms. CABG represents a viable treatment option in such cases, offering symptom relief and improved myocardial perfusion. However, the decision to pursue CABG should be made based on careful consideration of the patient’s symptoms, the extent of bridging, associated coronary artery disease, and the overall cardiovascular risk profile. Close collaboration between cardiologists and cardiac surgeons is essential to ensure optimal patient outcomes.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/Conflicts of Interest
The authors declare they have no conflicts of interest
Corresponding Author
Jerry Fan, MD
2401 S. 31st St Division of Cardiology
Temple, Texas 76504
Email: jerry.d.fan@gmail.com