Introduction
Heart failure (HF), a chronic condition with increasing prevalence as individuals age, causes significant morbidity and mortality and drives healthcare utilization and expenditure.1 Mortality rates approach 50% in the first five years following diagnosis.2 Estimates put the total costs for HF in 2012 at $30.7 billion, with two-thirds attributed to direct costs.3 There is a need for improved disease management using novel approaches to HF care.
Cognitive impairment affects as many as 80% of HF patients.4,5 When those patients have an abnormal Mini-Cog, a brief cognitive screening tool indicating cognitive impairment and possibly dementia, there is an associated increased risk of 30-day hospital readmission.6,7 Cognitive decline affects performance on instrumental activities of daily living (IADL) tasks earlier than for basic activities of daily living (ADL).8 Furthermore, proxies who know the patient well, such as caregivers, may offer more accurate IADL estimates, especially in cognitive impairment.9
As part of a Quality Improvement (QI) project, we explore the feasibility of determining patient- and proxy-reported functional abilities of older patients with heart failure and use the information from those assessments to inform discussions about post-hospitalization care plans. Older HF patients with cognitive impairment, as determined by Mini-Cog screening, would likely assess themselves as higher performing on functional abilities than that reported by their proxies. The discordance between self and proxy-reported functional ability performance would correlate with the patient’s insight into their level of cognitive impairment, giving us information to engage proxies in discharge care plans proactively.
Methods
As part of a QI initiative, community-dwelling individuals 60 years of age and older admitted to the cardiology inpatient service at a major urban academic hospital between December 2016 and May 2017 were assessed. Patients included those with an admission diagnosis of HF, who did not have delirium and whose Mini-Cog performance on admission indicated cognitive impairment. Patients and their proxies completed the Assessment of Living Skills and Resources Revision 2 (ALSAR). We excluded patients who declined the MoCA or ALSAR assessment from this analysis. Patients had access to standard hospital interpreter services as applicable and were able to complete screening evaluations with or without visual aids as applicable. The data collection process was deemed to be part of the standard of clinical care by the local IRB and not subject to research determination.
Measures
Demographic data collected from medical charts provided patient age and other characteristics. We defined subgroups by age and gender. Age was dichotomized according to the median.
Cognitive Impairment
We employed a two-step process with a more comprehensive assessment of those who demonstrated impairment on standard nurse-administered Mini-Cog. The Mini-Cog is a short cognitive test used as an initial screen for cognitive impairment.10 It consists of a 3-item recall and a clock drawing test (scores less than 3 indicate a higher likelihood of cognitive impairment).10 The admitting nurse administered the Mini-Cog within 24 hours of admission as part of the standard of care. A co-managing geriatrician performed a standard comprehensive geriatric assessment within 72 hours of admission, including the Confusion Assessment Method (CAM) to screen for delirium and the Montreal Cognitive Assessment (MoCA) to further screen for cognitive impairment if patients screened negative for delirium.11 The CAM includes four features that have the greatest ability to assess for the presence or absence of delirium.11 We excluded those for whom the CAM indicates possible delirium, which were performed simultaneously as Mini-Cog. The geriatrician-administered MoCA assesses cognitive performance using a 30-item tool with greater sensitivity for mild cognitive impairment and executive dysfunction than the Mini-Cog.12 In contrast to the Mini-Cog, which takes less than 5 minutes, MoCA administration takes an average of 10 minutes.12,13 A MoCA score below 26 points indicates cognitive impairment, with lower scores suggesting more severe impairment.13
Functional Assessment
We assessed performance on daily functional tasks using ALSAR. This instrument assesses the subject’s ability to perform everyday executive function tasks for independent living and associated resource availability to complete the task successfully. To perform executive function tasks, individuals need to combine skills that focus attention, recall, and ordering of skills to complete them, like instrumental activities of daily living (IADL) measured by the ALSAR. The ALSAR has been validated in older populations and is associated with the risk of needing more structured living arrangements, nursing home placement, hospitalization, and death.14–16 ALSAR comprises 11 tasks, including 8 IADLs and 3 other activities (reading, leisure, and home maintenance). Each task is assigned a “skill” and “resource” level. Skill levels are scored on a scale of 0-2, where “0” represents a task that is completed independently and consistently. Resource levels are scored on a scale of 0-2 where “0” represents consistent resource availability.16 The combination of scores for each task under both “skills” and “resources” (Skill-Resource) determines the task risk score, the scale of which is from 0-4. Higher scores indicate a higher risk of failing to complete a task. The ALSAR score is the sum of the 11 tasks (range 0-44). Differences between patient and proxy ALSAR scores were calculated by subtracting the total patient ALSAR score from the total proxy ALSAR score and represented as “ALSAR difference.” The complete scoring tool can be seen in the appendix.17
Statistical methods
We used the Pearson correlation to evaluate the association between MoCA and ALSAR difference. The Mann-Whitney U test was used for unpaired comparisons, and Wilcoxon Signed-Ranks test for paired comparisons. Chi-square test was used to compare frequencies for dichotomous variables. Data was analyzed with Social Science Statistics (https://www.socscistatistics.com/) and presented as median (IQR). The threshold for statistical significance was set at ɑ=0.05 using 2-tailed tests.
Results
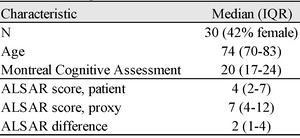
We report on 30 of 32 consecutive patients who met inclusion criteria, with two exclusions for missing MoCA assessments. The analytic sample had a median age of 74 years, 13 (42%) were female, and there were no differences in baseline characteristics between male and female participants. Age dichotomized across the sample median was not associated with patient ALSAR or proxy ALSAR scores. Patients older than the sample median had lower MoCA scores (p<0.01). In the overall group, the median patient ALSAR score of 4 (IQR 2-7) differed from the median proxy ALSAR score of 7 (IQR 4-12) (p<0.01; Table 1). The ALSAR difference was always zero or greater; therefore, proxies always scored patients the same as or higher (less independent) than patients who scored themselves on ALSAR. MoCA scores inversely correlated with ALSAR difference (r = -0.58, P<0.01; Figure 1).
Discussion
Those with higher MoCA scores more closely matched proxy estimated ALSAR scores, while more significant ALSAR differences corresponded with lower MoCA scores among hospitalized HF patients with cognitive impairment (Figure 1). Patients with cognitive impairment or dementia, a feature common in HF, struggle with complex executive function tasks such as medication management.18 Past work has shown the importance of impaired cognitive function determined by Mini-Cog in predicting poor post-hospitalization outcomes among HF patients.7 In a HF population with significant cognitive impairment, as suggested by failure to complete a Mini-Cog assessment, this pilot study correctly is an attempt to explore some of the variance in that population as viewed through the lens of the patient-proxy dyad interpretation of patient independence.
ALSAR scores range from 0-44, with scores near 0 representing a low risk for dependence and 44 representing a high risk for dependence on others for executive function tasks. In our population, patients’ composite ALSAR scores had a median (IQR) of 4 (2-7) while proxies scored 7 (4-12), overall, representing some, albeit relatively low risk for dependence.17 ALSAR difference in our population showed a median (IQR) of 2 (1-4) (Table 1). We suspect that even a small amount of dependence on IADL in individuals who also have an abnormal Mini-Cog may serve as a reveal for more significant underlying debility that can go unrecognized until, for example, hospital readmission occurs.7
Previous work shows decreasing agreement on IADL comparing assessments of patients compared to proxies along strata of worsening cognitive function. From the Canadian Study of Health and Aging, patients with increasing severity of dementia rated themselves as more independent than their proxies, and the level of agreement decreased incrementally among mild dementia and moderate dementia relative to controls.19 Another population from an outpatient geriatric clinic shows lower patient-proxy concordance of IADL reports among patients who scored worse on a cognitive assessment; those patients rated themselves as more independent than their proxy ratings.20 Similarly, MoCA scores were inversely associated with ALSAR difference in our population, which can be interpreted as greater patient-proxy agreement with better cognitive function. Interestingly, while all patients in this study met the criteria for cognitive impairment on the Mini-Cog, six patients had normal MoCA scores (Figure 1), and three showed an ALSAR difference greater than zero. Those cases can represent a subset of patients whose needs for task support might remain unrecognized by healthcare providers and the patient-proxy dyad. We hypothesize that patients like those in our sample who score well on MoCA may inspire false confidence in their abilities, whether self-assessed or by a caregiver proxy, which deserves further study. Efficiently identifying such persons at risk benefits from proxy input for reliable estimates of functional capacity, which could be achieved through measuring ALSAR difference.15
Proxies may also underestimate how much support is needed for the patient, especially given the complexities of HF self-management and the high rehospitalization rate among those who fail the Mini-Cog screen.7 Although prior research shows decreased agreement between patient and proxy IADL assessments with worse cognitive impairment, more extensive studies are warranted in confirming and contextualizing our finding of greater ALSAR difference associated with worse cognitive impairment as determined by MoCA.19,20 Additional studies are needed to determine to what extent ALSAR difference translates to added risk for rehospitalization and poor post-hospitalization outcomes.
Limitations
These data are derived from a single academic center. There may be variability in MoCA scoring related to a delay until the MoCA was performed up to 48 hours after admission. CAM screening was performed after Mini-Cog but prior to MoCA, which may confound the Mini-Cog interpretation. A large sample size is needed to confirm our findings. Further support for clinical implications, their extent, and the ALSAR difference value corresponding to clinical risk could also be sought through larger and prospective trials. Larger trials could also facilitate the determination of ALSAR domains most closely associated with clinically significant risk, enabling leaner screening tools in this population. We did not characterize the proxy relationship to the patient to evaluate the impact of that relationship status and its effect on the discrepancy, nor did we assess for cognitive impairment among proxies; our study presumes proxies’ familiarity with patients’ task performance. We do not have data on how many patients screened out of this pilot because of positive CAM, which would assist in clinical application. Finally, our study retained participants with “normal” MoCA scores for analysis acknowledging that it may conflict with the “abnormal” Mini-Cog results. In addition to testing slightly different aspects of cognition, Mini-Cog is a more efficiently applied tool with a lower threshold for adoption in clinical practice. For our purposes, MoCA aids in further stratifying cognitive function among patients who already met the criteria for cognitive impairment through Mini-Cog, given the association of “abnormal” Mini-Cog with heart failure hospital readmissions.7 Future research may include simultaneous Mini-Cog and MoCA screening to estimate implications of “abnormal” results from either or both tests.
Conclusions
Our study showed that MoCA scores correlate with ALSAR difference in an older inpatient HF population with cognitive impairment. ALSAR difference identifies skill deficits at risk of going missing if caregivers or proxies are not engaged in this population; the ramifications of missing these deficits could be inferred from prior studies showing the burden of disease cognitive impairment represents in HF populations and rehospitalization and mortality risk among HF patients with cognitive impairment.7,18 Measuring ALSAR differences in routine clinical practice may empower healthcare teams to create more robust patient-centered post-hospitalization support plans and may increase caregiver or proxy engagement. Patient and proxy ALSAR assessment that suggests functional independence in individuals who fail cognitive screens, such as the Mini-Cog, may deserve a formal, direct observational clinical assessment of executive function. This would ensure deficits in functional independence are not missed due to a lack of insight by patients or caregivers among those patients who can more easily cover for their deficits.
ALSAR difference is a novel and efficient metric reflecting the difference between patient- and proxy-reported functional ability, which deserves further investigation. Future studies could include in their design direct clinical observation for IADL performance and be powered such that individual tasks of the 11 tested in ALSAR can be associated for their specific relevance to clinically significant outcomes, thereby providing clinical context to ALSAR differences between proxy, patient, and clinical observer, and drive actionable interventions.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgment
We acknowledge support for this pilot project by University Hospitals Cleveland Medical Center.
Disclosures/Disclaimer
The authors declare they have no conflicts of interest. The views expressed herein are those of the authors and do not necessarily reflect the views of University Hospitals Cleveland Medical Center.
Corresponding Author
Mriganka Singh, M.D.
Division of Geriatrics & Palliative Care
Warren Alpert School of Medicine at Brown University
Suite 438, Physicians office Building
110 Lockwood Street, Providence, RI 02093 USA
Fax: 401-444-3397
Tel: 401-444-5248
E-mail: mriganka_singh@brown.edu