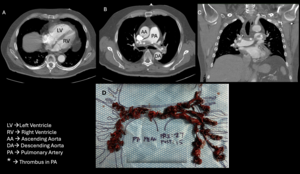
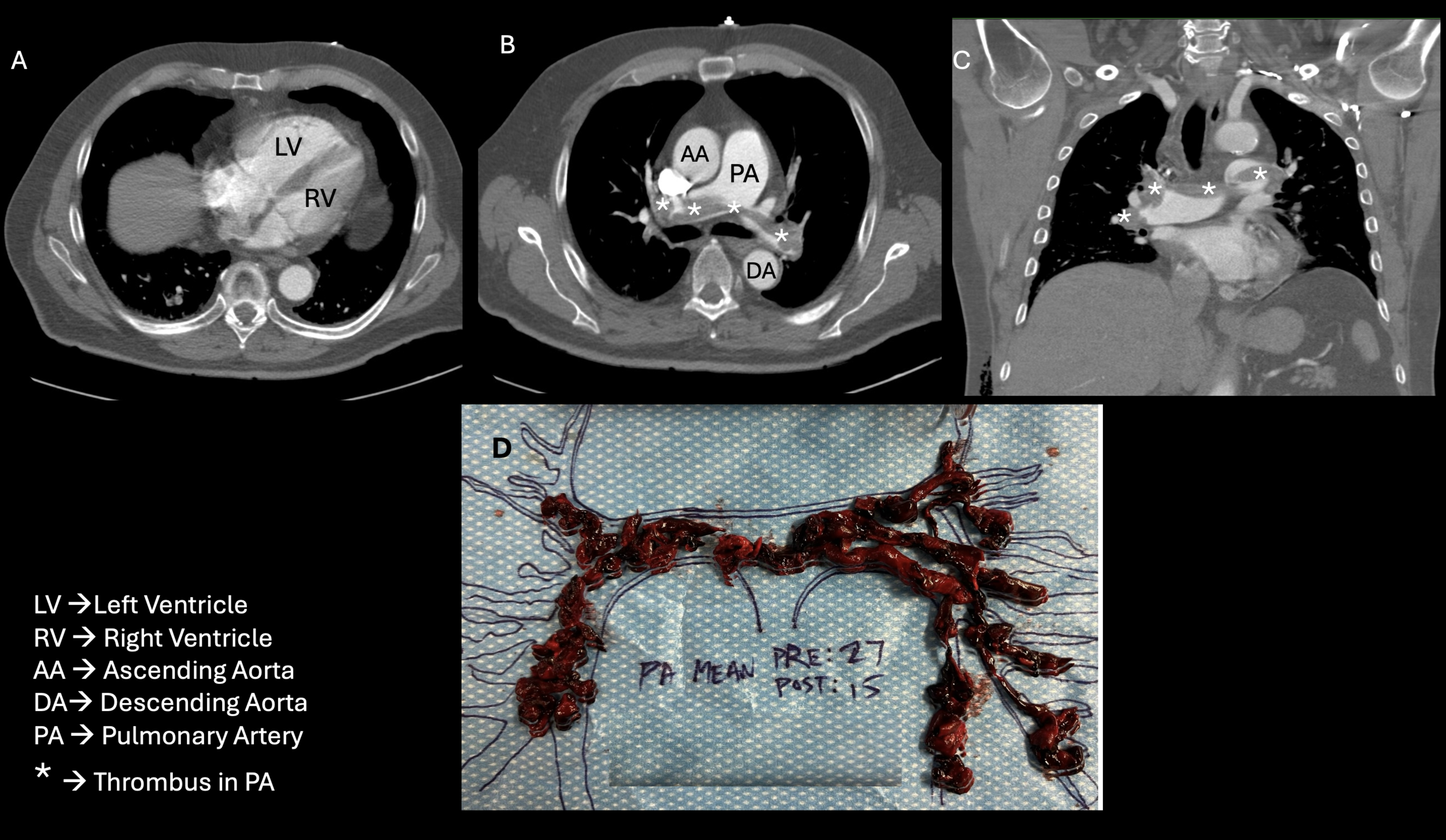
A 77-year-old man with history of recurrent bilateral leg deep vein thrombosis (DVT), and treated right upper extremity cutaneous melanoma, presented with chest pain four days after robotic assisted prostatectomy and pelvic lymphadenectomy for recently diagnosed Gleason 4+5 acinar cell adenocarcinoma of his prostate.1 The patient was diagnosed with his first right leg DVT eight years ago after an orthopedic surgery and a second left leg DVT during a recent COVID-19 infection. He was found to have a third DVT of his left leg four months prior to current presentation. He was treated with direct oral anticoagulants for three to six months after the first two clotting episodes and was treated with enoxaparin for three months for the most recent event. He stopped anticoagulation at the time of his prostate biopsy, which took place three weeks prior to his prostatectomy and did not resume anticoagulation immediately post operatively. In the days leading up to his admission, he developed worsening dyspnea on exertion and chest pressure. In the emergency department (ED) he was noted to be in moderate respiratory distress, with oxygen (O2) saturations of 92% on two liters of supplemental O2 but dropped to under 90% when on room air. His vitals showed a blood pressure of 104/64mmHg and heart rate of 89 beats per minute. Physical exam was remarkable for a loud pulmonary component of his second heart sound over the second right intercostal space. His right calf was slightly swollen in comparison to his left side. The dorsalis pedis and posterior tibial pulses were palpable bilaterally. His laparascopic surgical sites were clean, dry and not oozing. His labs showed a mildly elevated white cell count at 12.9 x109/L and an elevated Troponin I 216 ng/ml (normal < 14ng/L). Computed Tomography Angiogram (CTA) of his chest revealed a large saddle pulmonary embolus (PE) with clot involving all five lobes of the lungs and an elevated RV/LV ratio of 1. A new right ventricular (RV) wall-motion dyskinesis with apical sparing (positive McConnell sign) was noted on bedside echocardiogram.2 Bilateral lower extremity ultrasound showed an acute DVT in the right posterior tibial and peroneal veins, and a chronic DVT in the left popliteal, posterior tibial and peroneal veins. In the ED, he was placed on a heparin drip and underwent an emergent catheter directed aspiration pulmonary thrombectomy as his recent surgery precluded the administration of systemic thrombolysis. He tolerated the procedure well despite his large clot burden (Figure 1). His pre-embolectomy mean pulmonary artery pressure decreased from 27 mmHg to 15 mmHg, and repeat echocardiogram revealed normal RV size and improved contractility (Figure 2).
This case demonstrates the clinical conundrum hospitalists and perioperative physicians face when confronted with a life-threatening acute pulmonary emboli in the post-operative setting. Our case was complicated by a high bleeding risk, a recent thrombotic event, as well as a recent second cancer diagnosis. In addition, surgery close to the inferior vena cava bed raises the risk of venous thromboembolism significantly. Given his dire prognosis, an emergent decision about life-saving treatment was made. His simplified pulmonary embolism severity index (sPESI) was calculated as 2, with a point each for room air arterial oxygen saturation <90% and a history of cancer placing him at high risk of death.3 Additional factors portending a poor outcome included a very high clot burden, new worsening right ventricular function, and age >65. In assessing his suitability for thrombectomy, all risk factors were considered and involved a multidisciplinary team-based approach (Hospitalist, Interventional Cardiologist, Intensivist, and ICU Team). Of the absolute and relative contraindications for thrombolysis, he had two relative contraindications with his age >75 and a recent invasive procedure on a highly vascular anatomic area, the prostate. Ultimately, the consensus decision to proceed was based on a higher risk of death from large saddle embolism and the risk of life-threatening bleeding if systemic thrombolytics were used. Additionally, the patient’s systolic blood pressure was dropping, and prompt clot removal was deemed essential. Catheter Directed Aspiration Thrombectomy (CDT) was the treatment modality chosen and avoided the use of thrombolytics.4–6 Using a femoral approach, an intravascular catheter was introduced and threaded towards the pulmonary artery. When the probe tip reached the clots, aspiration was performed into this large bore catheter attached to the aspiration syringe (Figure 1D).7
The efficacy and safety of this technique was evaluated in the FlowTriever for Acute Massive pulmonary Embolism (FLARE) study (106 intermediate- risk PE patients) which was a multicenter and prospective study showing generally favorable results – 40% of patients did not require ICU stays, and only six major adverse events in four patients were reported. Only one non-device related death occurred in a patient with metastatic breast cancer. The rate of major bleeding was only 0.9%. Of note, the study excluded high risk PE patients who had undergone surgery or in whom thrombolytics were contraindicated.8
Some limitations of this specific aspiration thrombectomy method described in the literature are the catheter size and relative inflexibility, which makes it harder to advance into smaller-sized distal vessels. Operators’ technical expertise and experience with manipulating the catheters are generally needed for optimal results.9 Another serious complication reported is pericardial microperforation, which may progress to cardiac tamponade.10 The ongoing FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH) registry has enrolled 734 patients thus far and currently lists a <1.0% all-cause mortality.11,12
Percutaneous Aspiration thrombectomy is an emerging and promising option for the treatment of acute PE, particularly in intermediate-high-risk groups with contraindications to systemic thrombolysis, as in our case. The goal of timely endovascular treatment is to facilitate the improvement of pulmonary blood flow and RV recovery without the complications of thrombolytics. Larger studies and prospective data are needed to evaluate long-term safety and efficacy.
Author Contributions
All authors have reviewed the final manuscript prior to submission. All the authors have contributed significantly to the manuscript, per the International Committee of Medical Journal Editors criteria of authorship.
-
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
Drafting the work or revising it critically for important intellectual content; AND
-
Final approval of the version to be published; AND
-
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures/Conflicts of Interest
The authors declare they have no conflicts of interest
Corresponding author
Michael E. Lazarus, MD
Professor of Clinical Medicine
757 Westwood Plaza, Suite 7501C, Los Angeles, CA 90095
Tel: 310-267-9645
_of_heart._pre-procedure_(left)_in_diastole_showi.jpg)
_of_heart._pre-procedure_(left)_in_diastole_showi.jpg)